Overview
The fall armyworm (Spodoptera frugiperda) is a plant pest that can damage a wide variety of crops. The larvae predominantly feed on crops and pastures from the Poaceae (grass) family, in particular maize, but also sorghum, and forage grasses. It has also been noted on other grass crops including cereals but these are considered secondary hosts. The pest can also feed on non-grass crops such as cotton, vegetables and some fruit crops. Fall armyworm is known for its ability to disperse and migrate long distances, which enables it to exploit new habitats and expand its range when favourable host plants and climatic conditions are present.
Fall armyworm is a moth native to the American tropics. It has become a worldwide pest and was first recorded in Australia in January 2020 on two Torres Strait islands, followed by discoveries in Queensland (February 2020), and the Northern Territory (March 2020).
Fall armyworm was first detected in Western Australia in Kununurra, in the Kimberley region, in March 2020.
The Consultative Committee on Emergency Plant Pests (CCEPP) met on 24 February 2020 and concluded that fall armyworm is not technically feasible to eradicate—for National Management Group decision.
It has since been detected at other locations in northern Western Australia and in some more southerly areas of the state, most recently in a faba bean crop at Cuballing in July 2024.
The expected distribution of fall armyworm in Australia has been modelled based on weather data over the past 30 years. The following map indicates areas where the insect is likely to survive year-round as well as areas where low winter temperatures prevent permanent presence.
Areas with suitable hosts would likely be invaded by this strong flying moth as conditions become suitable. This is the case in the tropical and sub-tropical areas elsewhere in the world where the insect survives year-round and moves both north and south as conditions become more suitable.
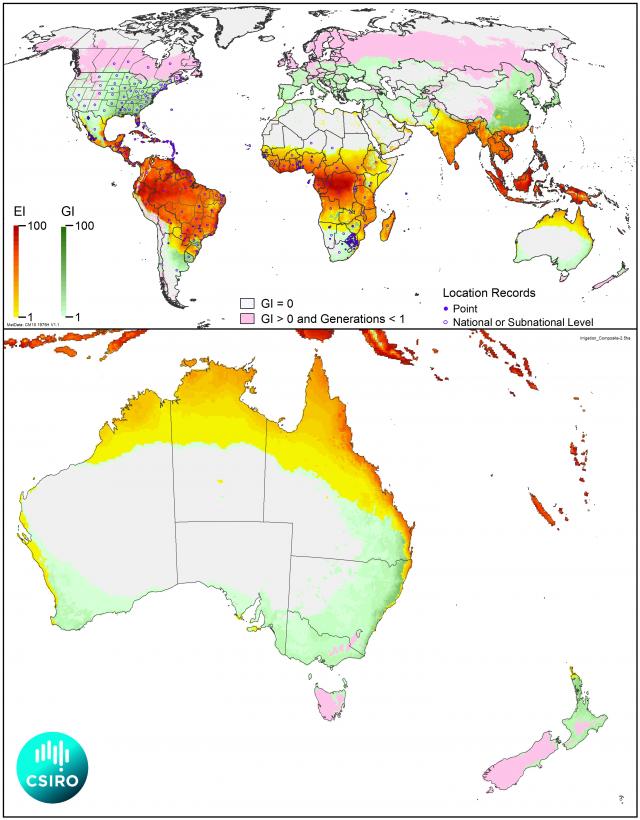
The global and Australian potential distribution of fall armyworm (Spodoptera frugiperda) modelled using CLIMEX above in the yellow-red-shaded areas indicate relative climatic suitability for establishment of persistent populations. The green-shaded areas suggest climatic suitability for seasonal migration during the warmer months. Pink areas are predicted to be unable to support a full generation of the moth.
Learn more about fall armyworm:
Surveillance and reporting
The fall armyworm (Spodoptera frugiperda) is a plant pest that can damage a wide variety of crops. The larvae predominantly feed on crops and pastures from the Poaceae (grass) family, in particular maize, but also sorghum, and forage grasses. It has also been noted on other grass crops including cereals but these are considered secondary hosts. The pest can also feed on non-grass crops such as cotton, vegetables and some fruit crops. Fall armyworm is known for its ability to disperse and migrate long distances, which enables it to exploit new habitats and expand its range when favourable host plants and climatic conditions are present.
In October 2019, two pheromone traps were set up in the agricultural area of the Ord River Valley, near Kununurra, Western Australia, as part of the national Northern Area Quarantine Surveillance program.
After the detection of fall armyworm in the Torres Strait and Queensland, DPIRD and Ord River District Co-operative Ltd (ORDCO) established an additional 22 traps established in the Ord Valley.
The first detection of fall armyworm in Western Australia occurred in Kununurra in March 2020 and was confirmed on 1 April 2020. This was followed by detections of egg masses and larvae in maize and sorghum crops in Kununurra during April and additional detections of adults in various traps across the Ord Valley.
DPIRD in collaboration with growers and agronomists expanded the surveillance program statewide.
During March 2020, fall armyworm adults were collected in traps, and larvae were detected in the Broome area in forage sorghum, maize, sweet corn and Rhodes grass. Fall armyworm adults were found in Carnarvon in April 2020.
Fall armyworm adults were detected in Geraldon and Northam in pheromone traps, but there was no evidence of reproduction or establishment. Larvae were found in sweet corn crops north of GinGin but fall armyworm did not establish year round.
Since 2022, growers and agronomists have been advised to conduct surveillance in the areas where fall armyworm does not establish year-round.
Fall armyworm caterpillars were found in a faba bean crop in Cuballing in July 2024.
Growers and agronomists are encouraged to monitor for fall armyworm larval through crop sampling with a sweep net and if interested, can also set up a pheromone trap for adult moths.
Information on how to set up traps is provided in DPIRD's Fall armyworm surveillance trapping training manual.
For more details on how to set up a pheromone trap for fall armyworm moths and monitor for larvae, watch the webinar conference presentation delivered by DPIRD senior biosecurity officer David Cousins on 9 April 2020. Alternatively, see the PowerPoint presentation here.
Reporting
Monitoring the spread of this insect across Western Australia will assist DPIRD to determine the year-round distribution and migration patterns of fall armyworm.
If you suspect fall armyworm in your crops make a report using the PestFacts WA Reporter app.
Biology
The fall armyworm (Spodoptera frugiperda) is a plant pest that can damage a wide variety of crops. The larvae predominantly feed on crops and pastures from the Poaceae (grass) family, in particular maize, but also sorghum, and forage grasses. It has also been noted on other grass crops including cereals but these are considered secondary hosts. The pest can also feed on non-grass crops such as cotton, vegetables and some fruit crops. Fall armyworm is known for its ability to disperse and migrate long distances, which enables it to exploit new habitats and expand its range when favourable host plants and climatic conditions are present.
Life cycle and development
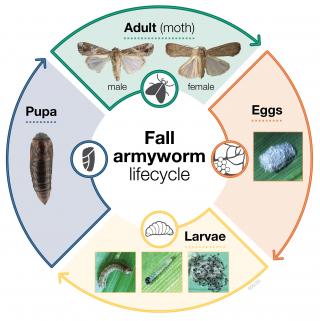
Fall armyworm has four life stages: egg, larvae (caterpillar), pupae and adult (moth). At its ideal temperature (28º Celcius (C)), fall armyworm can complete its life cycle within 30 days, from egg laying to the emergence of adult moths.
After mating the female moth will lay eggs in clusters of 50 to 200 eggs, with clusters covered in hairs or scales. Female moths lay most of their eggs within four to five days of mating but can continue laying for up to two weeks. The eggs hatch within two to four days after being laid on the lower leaf surfaces.
After hatching, fall armyworm larvae complete five to six growth stages and reach maturity in 14 to 22 days. Once mature, larvae drop to the ground, where they pupate for around eight to nine days in warmer months, and 20 to 30 days in cooler areas, before emerging as a moth.
Fall armyworm do not diapause (suspend development) during any stage. They live year-round in places where there are available hosts and favourable temperatures. The ideal range for development is 23-30ºC. The insect can continue to to develop slowly below this range but do not survive year-round where temperatures fall below 9-12⁰C and where frosts occur.
Dispersal and migration
Fall armyworm is well known for its capacity to disperse within a crop locally and migrate over large distances. The adult moths are good flyers and can travel hundreds of kilometres in a short period of time. In North America, fall armyworm can survive year-round in southern Florida and Texas. As temperatures rise, populations build up and moths migrate northward along the eastern seaboard as far north as Canada.
Fall armyworm has been accidentally introduced to other parts of the world and was found in the African continent in 2016, the Indian subcontinent in 2018 and was found in Southeast Asia and China in 2019.
For Western Australia, this means that fall armyworm will establish in areas in the north with favourable conditions and host plants and migrate southward as host plants and climatic conditions remain suitable. If conditions are favourable, it could complete a few generational cycles in southern areas.
In addition to the adults, fall armyworm larvae also disperse. The neonates spin a silk thread that they use to balloon away from the egg mass to nearby host plants. Larvae of all ages are able to quickly crawl from one host plant to another.
This means both adults and larvae are able to move quickly within crops and from one crop to another or to nearby host plants after harvest.
Natural enemies
A number of natural enemies present in crops that attack the eggs, larvae and pupae of other lepidopteran pests are likely to also attack fall armyworm in the same crops.
Generalist predators, such as spiders, beetles, ants, sucking bugs and predatory wasps, will likely reduce the numbers of eggs, larvae and pupae. The adults and pupae may be fed upon by birds and other animals. A study conducted in Georgia, United States, demonstrated that 60 to 90 per cent of pupae were consumed by predators. Soil pathogens, such as nematodes, may infect the pupae and drive numbers down.
Overseas, egg parasitoids are very important for biological control. Parasitoids such as Trichogramma pretiosum have been shown to reduce the number of larvae hatching from the egg masses and thereby reduce damage to the host plant. These parasitoids have been easily mass-reared and are available in Australia where they have been used for management of Heliothis and other moth pests. They have been found parasitising fall armyworm eggs in northern WA. Other parasitoids, such as Telenomus remus, are also present in Australia and reduce fall armyworm numbers overseas. This parasitoid has been found in fall armyworm in the Northern Territory.
Larval parasitoids have also reduced fall armyworm numbers overseas. Cotesia marginiventris, which is present in Australia, is an important natural enemy in several countries. Cotesia ruficrus and other species have been found attacking fall armyworm in Western Australia.
Identification
The fall armyworm (Spodoptera frugiperda) is a plant pest that can damage a wide variety of crops. The larvae predominantly feed on crops and pastures from the Poaceae (grass) family, in particular maize, but also sorghum, and forage grasses. It has also been noted on other grass crops including cereals but these are considered secondary hosts. The pest can also feed on non-grass crops such as cotton, vegetables and some fruit crops. Fall armyworm is known for its ability to disperse and migrate long distances, which enables it to exploit new habitats and expand its range when favourable host plants and climatic conditions are present.
Fall armyworm have four life stages: eggs, larvae (caterpillar), pupae and adult (moth).
Identifying fall armyworm from the eggs, pupae and adult moths is very difficult. The larvae have key identification features growers can use, however many larvae present in Western Australian crops look similar to fall armyworm.
For a detailed look at the identification characteristics of fall armyworm, watch the webinar conference presentation or read the PowerPoint presentation by DPIRD senior laboratory scientist Melinda Moir, delivered on 9 April 2020 to growers, agronomists and stakeholders.
Eggs
Fall armyworm eggs are pale yellow coloured and laid as an egg mass, which often contains 100–200 eggs. Egg masses are laid on leaves and covered with a layer of hairs and scales shed by the female moth.
Larvae
The appearance of larvae changes as they develop through the life cycle. When larvae first hatch from the eggs, they are light coloured with a larger dark head.
As they develop, the body of fall armyworm larvae become darker with white lengthwise stripes. They also develop dark spots with spines. The colour can vary from light green to a dark brown and black.


Key features found on larvae can be used for identification. For more details, see the Fall armyworm larval identification guide.

Adults
Identification of fall armyworm moths is very difficult and requires an expert. There are many other species of moths that look similar to fall armyworm.
The adult moths are 32-40mm in length from wing tip to wing tip, with a brown or grey forewing and a white hind wing. Male fall armyworm moths have more patterns on their wings than the females. Males also have a distinct white spot on each of their forewings.
What else could it be?
Table A lists pest moths in the crop that could be confused with fall armyworm (as at 20 May 2020)
| Common name | Species name | Crops in which it is found |
| Cluster caterpillar, tobacco cutworm | Cotton, maize, sorghum, pastures, hay | |
| Beet armyworm | Beets, asparagus, beans, peas, cabbage, pepper, tomato, lettuce, celery, strawberry, eggplant, sugar beet, alfalfa, cole crops, potato, cotton, cereals, oilseeds, tobacco, flowers and weed species | |
| African armyworm | Cereals, maize, rice, sorghum, sugarcane, and pasture grasses, especially Cynodon and Pennisetum species | |
| Native budworm | Maize, cotton, canola, pulses, sunflower, flax, pasture legumes, fruit and vegetable crops | |
| Corn earworm, cotton bollworm | Major hosts: maize, tomato, cotton, pigeon pea, chickpea, rice, sorghum, and cowpea. Other hosts include: groundnut, okra, peas, field beans, soybeans, lucerne, Phaseolus spp., other Leguminosae, tobacco, potatoes and flax | |
| Lesser budworm | Cereals and lucerne | |
| Northern armyworm | Rice, maize, sorghum, wheat, sugarcane and wild grasses | |
| Common armyworm | Poaceae spp. (inc. cereals and grasses), pineapple, sweet potato and lucerne | |
| Sugarcane armyworm | Sugarcane, Poaceae spp. (inc. cereals and grasses) | |
| Southern armyworm | Poaceae spp. (inc. cereals and grasses), peas and flax | |
| Inland armyworm | Poaceae (inc. cereals and grasses) | |
| Black cutworm | Maize, crops and weeds |
Management overview
The fall armyworm (Spodoptera frugiperda) is a plant pest that can damage a wide variety of crops. The larvae predominantly feed on crops and pastures from the Poaceae (grass) family, in particular maize, but also sorghum, and forage grasses. It has also been noted on other grass crops including cereals but these are considered secondary hosts. The pest can also feed on non-grass crops such as cotton, vegetables and some fruit crops. Fall armyworm is known for its ability to disperse and migrate long distances, which enables it to exploit new habitats and expand its range when favourable host plants and climatic conditions are present.
Fall armyworm management has been a challenge worldwide. The key to effective management is early detection of the pest in the crop and regular monitoring to assess the population build up.
If fall armyworm becomes an important/consistent pest in a particular region, a pheromone trap can be deployed to check on the presence of moths as a warning of potential pest situations.
Larval monitoring is a more reliable method for determination of infestation.
Host-plant resistance and variations in production practices have been used quite successfully in other parts of the world. Integrated Pest Management that employs a variety of tactics will be essential to managing fall armyworm for the long-term.
Further research is needed to understand more about this insect, how it is going to impact the crops in Western Australia and even where and when it will become problematic. Management strategies will also be an important research area for DPIRD.
For an overview on managing fall armyworm in Western Australia, see the webinar conference presentation delivered by DPIRD senior research scientist Helen Spafford on 9 April 2020 to growers, agronomists and stakeholders. Alternatively, read the PowerPoint presentation.
Special considerations
Fall armyworms will feed on crops where other lepidopteran pests, such as cluster caterpillar, armyworms, bollworms and others, are already present and established. Many of the management strategies used for lepidopteran pests may also be effective for fall armyworm.
Pesticides are often the primary tool for management and many products are effective at reducing pest numbers. However, fall armyworm is reported to relatively quickly develop resistance to insecticides and insecticide use for fall armyworm may also change the dynamics of other pests in the system. It is therefore important to use insecticides judiciously. Where required, APVMA permits should be read in conjunction with the relevant product label for information on withholding periods and other critical comments.
Natural enemies are found suppressing fall armyworm populations around the world. There is a suite of natural enemies that attack pests similar to fall armyworm in the same crops in Western Australia. It is likely these natural enemies will also reduce fall armyworm numbers. If pesticides are used for fall armyworm management, it is advised that beneficial-friendly products are applied.
The following links provide information on scrop-specific fall armyworm management options:
- Management in maize and sweet corn
- Management in sorghum
- Management in grains, canola and pulses
- Management in horticulture - vegetables and fruit
Management in maize and sweet corn
Damage
Maize and sweet corn are among the more favoured host plants for fall armyworm. Larvae can reduce the yield of grain, forage and cobs by affecting plant establishment, damaging leaves and attacking cobs.
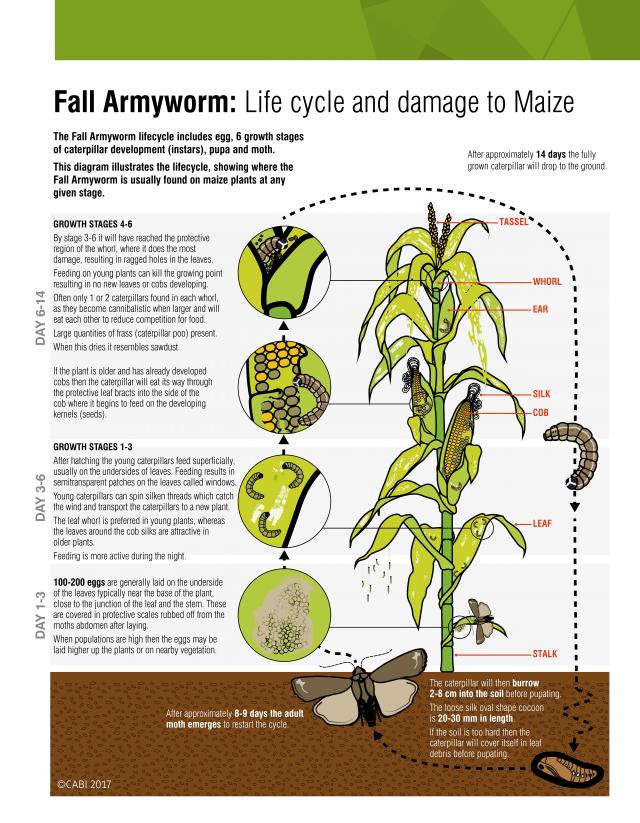
Plant establishment is most likely to be affected when advanced stage larvae move into a newly planted crop from adjacent areas of crops or weeds. Fall armyworm damage to young plants is similar to that of cutworms, where the plant is either cut off at ground level or killed by burrowing into the growing tip.
The usual source of infestation is from moths laying egg masses on young plants. Newly emerged larvae feed on leaves and remove only the green tissue, leaving leaf tissue behind, resulting in a ‘windowing’ effect.
As larvae increase in size, they remove chunks off the leaves. As plants grow, the larvae feed within the whorl (throat) of plants and are not seen during the day. They may emerge at night to feed. Their frass (droppings) may be visible near the leaf damage. Examination of leaves inside the whorl is needed to confirm the identity of the pest – other armyworms and ‘heliothis’ also infest maize, causing similar damage. Heavy infestations of fall armyworm result in a ragged appearance of plants. Fall armyworm larvae are cannibalistic and usually only one or two large larvae will occur within each whorl as the larvae develop.
Maize plants can tolerate some level of damage to leaves without affecting yield. The extent of damage depends on the timing and magnitude of fall armyworm infestation. This is incorporated in suggested action thresholds mentioned below.
Larvae also feed on the tassel, silks and cobs of maize/sweet corn plants. Damage to tassels is unlikely to affect yield, but larvae feeding on tassels may move to the silks and cobs. Feeding on silks may affect kernel fertilisation. Larvae are likely to feed on developing kernels in cobs by entering the cob from the side, unlike ‘heliothis’ larvae which usually enter cobs via the silks. Fall armyworm feeding on cobs results in some yield loss in maize crops but may cause significant loss through cosmetic damage in sweet corn crops for human consumption, where such cobs are rejected from market.
Monitoring
Since its establishment, DPIRD is continuing to monitor the presence of fall armyworm in the northern agricultural areas of the State especially in the Kimberley region where several favoured host crops, including maize, are grown and where fall armyworm populations are established year-round.
If there are indications of fall armyworm becoming a consistent pest in specific regions, a pheromone trap can be deployed in crops to check on the presence of moths as a warning of potential pest situations.
Growers and agronomists are suggested to start scouting as soon as maize/sweet corn seedlings emerge. Weekly checks thereafter are recommended. Check 20 consecutive plants (randomly selecting the first) from at least five locations across the crop, or 10 plants at 10 locations.
Regularly check and record the:
- Percentage of plants infested with fall armyworm larvae and the
- Characteristic damage:
- windowing indicates the start of an infestation, and
- gross leaf damage suggests the presence of older larvae.
- Accumulation of frass/excreta around the whorl.
A few days before tasselling, check for large larvae in the whorls, which will be pushed out when the tassels emerge. These larvae may attack young ears.
Continue to check for fall armyworm larvae until silks begin to dry. On very rare occasions, an infestation of armyworm has been an issue in maize after silking, causing considerable leaf loss, which may have affected yield by reducing the plants’ ability to fill out kernels.
When to take action
The action thresholds recommended for applying control measures for fall armyworm vary with the growth stage:
- At the seedling stage, if more than 5% of plants are cut.
- At the early whorl stage (knee high), if more than 20% of plants are infested.
- At the late whorl stage (shoulder high), if more than 40% of plants are damaged and live larvae are present.
- At the tasselling/early silking stage, in sweet corn, if more than 5% of plants are infested and in maize, if more than 20% of plants are infested.
Management approach
An integrated pest management (IPM) approach should be considered for protecting crops from infestations of fall armyworm.
Fall armyworm has entered an existing suite of pests and associated natural enemies, systems of production and pest management programs. Careful consideration needs to be given to any actions taken for fall armyworm control that may have adverse effects on management options already in place for other pests of maize or sweet corn, especially where natural control agents are used.
Natural enemies of other insects in the same group as fall armyworms already present in maize and sweet corn crops may also attack fall armyworm. There is already evidence of wasps parasitising fall armyworms in maize and sorghum in Kununurra. However, the effect of natural enemies with fall armyworm will become clearer as our experience with the pest accumulates.
An important management practice is to maintain farm biosecurity measures and implement good farm hygiene, and to remove alternative hosts such as weeds and volunteer crop plants, especially during periods, and in places, where fall armyworm would not easily be able to survive year-round.
Other cultural practices, such as trap cropping, may reduce fall armyworm numbers. Understanding the value of the various cultural practices for fall armyworm management that have been tested overseas will requires further study under Australian conditions.
In the Americas, Spodoptera frugiperda nuclear polyhedrosis virus (SfNPV) has been successful in significantly reducing the damage caused to maize crops, but this product is now available in Australia and can infect and kill young fall armworm larvae. The NPV that controls Helicoverpa larvae is not considered to be effective against fall armyworm.
Insecticides that contain the bacterium Bacillus thuringiensis (Bt) would most likely kill larvae of fall armyworm, especially if applied when larvae are small. However, considering this product need to be ingested and larvae feed in concealed areas, foliar applications of Bt products are not recommended for use in maize/sweet corn crops.
In the short term, insecticides are available to help protect crops from fall armyworm. However, this insect has a reputation for developing resistance to insecticides and the populations of fall armyworm found in Australia are already resistant to the Group 1 insectides and have reduced sensitivity to others. Resistance management strategies will therefore be required to maintain effectiveness of insecticides for controlling this pest.
Application of insecticide will be most effective if applied late in the day and into the night, when larvae become more active and emerge from protected areas of the plant.
Insecticides available for use against fall armyworms include those available under recently approved APVMA minor use permits. Also available for use in WA, are any insecticides registered for use on crops for control of other insects if those products are considered effective on fall armyworm and provided they are applied according to label details. See section 87 ‘Use in accordance with label’, page 53 of WA Health (Pesticides) Regulations 2011.
The following tables list the details of pesticides available with current permits in Australia for maize (Table 1) and sweet corn (Table 2), and their specified application rates. The permits should be read in conjunction with the relevant product label for information on withholding periods and other critical comments.
More detail on the permits is available from the information sheets found on the APVMA Portal. A direct link to each minor use permit PDF is provided in the tables below.
Note: New permits are regularly issued for fall armyworm control. Check the APVMA Portal for the most current information.
Table 1. List of current permits in Australia for maize crops (as at 20 May 2020)
| APVMA permit | Insecticide | Rate of product/ha | IRAC*/MOA** classification |
| alpha-cypermethrin 250g/L | 88-112 mL/ha | 3A | |
| alpha-cypermethrin 100g/L | 220-280 mL/ha | 3A | |
| alpha-cypermethrin 100g/L | 400 ml/ha | 3A | |
| methomyl 225g/L | 2000 mL/ha + wetter; see label | 1A | |
| zeta-cypermethrin 100g/L | 500 mL/ha | 3A | |
| chlorantraniliprole 350 g/kg | 70-90 g/ha | 28 | |
| Spinetoram 120g/L | 250-300 mL/ha | 5 | |
| Indoxacarb 150 g/L | 400-500 mL/ha | 22 |
*Insecticide Resistance Action Committee **Mode of Action
Table 2. List of current permits in Australia for sweet corn crops (as at 20 May 2020)
| APVMA permit | Insecticide | Rate of product/ha | IRAC*/ |
| spinetoram 120 g/L | 400 mL/ha | 5 | |
| chlorantraniliprole 200 g/L | 100 mL/ha | 28 | |
| emamectin benzoate 44 g/kg | 250 g/ha | 6 | |
| alpha-cypermethrin 100 g/L | 400 mL/ha | 3A | |
| methomyl 225 g/L | 2000 mL/ha + wetter; see label | 1A | |
| zeta-cypermethrin 100 g/L | 500 mL / ha | 3A | |
| methomyl 225 g/L | 1.5 - 2 L/ha | 1A | |
| methomyl 400g/kg | 0.84 – 1.13 kg/ha | 1A | |
| ememectin 17 g/L | 600-900 mL/ha | 6 |
*Insecticide Resistance Action Committee **Mode of Action
Important disclaimer
The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it.
Management in sorghum
Damage
Sorghum is among the more favoured host plants for fall armyworm. Larvae can reduce the yield of forage and grain by affecting plant establishment, damaging leaves and attacking sorghum heads.
Plant establishment is most likely to be affected if more advanced stage larvae move into a newly planted crop from adjacent areas of crops or weeds. In this situation, damage to young plants is similar to that of cutworms, where the plant is either cut off at ground level or killed when larvae burrow into the growing tip. Sorghum plants will compensate by producing other tillers but this slows the crop growth and may reduce yield.
The usual source of infestation is from moths laying egg masses on young plants. Newly emerged larvae feed on leaves and remove the green tissue only, resulting in a ‘windowing’ effect with the leaf epidermis left behind (see the photo below illustrating leaf damage on sorghum where the white areas on leaves are the result of feeding by early stage larvae). Fall armyworm larvae typically infest the whorl (throat) of sorghum plants, resulting in emerging leaves having large, irregular-shaped holes.
Other armyworm species and Helicoverpa armigera will cause the same type of damage in sorghum, so it is important to open the whorl to identify the larvae. Leaf damage to younger sorghum plants does not usually impact on plant growth and yield, unless pest pressure is high and defoliation severe. Heavy infestations result in a ragged appearance to plants. Fall armyworm larvae are cannibalistic and usually only 1 or 2 large larvae will occur within each whorl as plants develop.
As panicles emerge, fall armyworm can infest the heads and damage the developing grain. Like H. armigera, small fall armyworm larvae feed on the pollen and larger larvae feed on the developing grain. Managing infestations of fall armyworm before heads emerge will reduce the risk of this type of damage. Check for the infestation level in crops and the age of larvae prior to the emergence of sorghum heads.
Monitoring
If fall armyworm becomes an important/consistent pest in a particular region, a pheromone trap can be deployed to check on the presence of moths as a warning of potential pest situations.
Larval monitoring is a more reliable method for determination of infestation.
It is suggested that growers and agronomists start scouting as soon as sorghum seedlings emerge. Weekly checks thereafter are recommended. Check 20 consecutive plants (selecting the first randomly) from at least five locations or 10 plants at 10 locations across the crop.
Regularly check and record:
- Percentage of plants infested with fall armyworm larvae, and the
- Characteristic damage:
- windowing indicates the start of an infestation
- gross leaf damage the presence of older larvae, and
- Accumulation of frass/excreta around the whorl.
Confirm the presence of fall armyworm on the damaged plant. The damage could be caused by another species.
A few days before panicle emergence, look for larvae in the whorls, which will be pushed out when the panicles emerge. These larvae will attack the developing grain. The infestation of fall armyworm in sorghum heads may be assessed by tapping heads over a container. On very rare occasions, with heavy infestations, when grain is hardening, fall armyworm may continue attacking foliage. Extensive leaf loss may affect yield by reducing the plants’ ability to ripen the grain.
When to take action
Economic thresholds have not yet been developed for sorghum grown in Australia. The action thresholds recommended overseas for applying control measures for fall armyworm vary with the growth stage:
- At the seedling stage, if more than 10% of plants are cut.
- At the early whorl stage (knee high), if more than 30% of plants are infested.
- At the late whorl stage (shoulder high), if more than 40% of plants are damaged and live larvae are present, and
- At the panicle emergence stage, if more than 5-10% of plants are infested.
Management
An integrated pest management (IPM) approach should be considered for protecting crops from infestations of fall armyworm.
Fall armyworm has entered an existing suite of pests and associated natural enemies, systems of production and pest management programs. Careful consideration needs to be given to any actions taken for fall armyworm control that may have adverse effects on management options already in place for other pests of sorghum, especially where natural control agents are used.
Natural enemies of other insects in the same group as fall armyworms already present in sorghum may also attack fall armyworm. There is already evidence of wasps parasitising fall armyworms in sorghum and maize crops in Kununurra. However, the effect of natural enemies with fall armyworm will become more clear as our experience with the pest accumulates.
An important management practice is to maintain farm biosecurity measures and implement good farm hygiene, and remove alternative hosts such as weeds and volunteer crop plants, especially during periods, and in places, where fall armyworm would not easily be able to survive year-round.
Other cultural practices, such as trap cropping, may reduce fall armyworm numbers. Understanding the value of the various cultural practices for fall armyworm management that have been tested overseas will requires further study under Australian conditions.
In the Americas, Spodoptera frugiperda nuclear polyhedrosis virus (SfNPV) has been successful in significantly reducing the damage caused to maize crops, but this product is now available in Australia and can infect and kill young fall armworm larvae. The NPV that controls Helicoverpa larvae is not considered to be effective against fall armyworm.
Insecticides that contain the bacterium Bacillus thuringiensis (Bt) would most likely kill larvae of fall armyworm, especially if applied when larvae are small. However, considering they need to be ingested and the concealed areas where larvae feed, foliar applications of Bt products are not recommended for use in sorghum crops.
In the short term, insecticides are available to help protect crops from fall armyworm. However, this insect has a reputation for developing resistance to insecticides. Resistance management strategies will therefore be required to maintain effectiveness of insecticides for controlling fall armyworm.
Application of insecticide will be most effective if applied late in the day and into the night, when the larvae become more active and emerge from the protected areas of the plant.
Insecticides available for use against fall armyworms include those available under recently approved APVMA minor use permits. Also available for use in WA, are any insecticides registered for use on crops for control of other insects if those products are considered effective on fall armyworm and provided they are applied according to label details. See section 87 ‘Use in accordance with label’, page 53 of Western Australia’s Health (Pesticides) Regulations 2011.
Table 1 lists the details of pesticides available with current permits in Australia for sorghum and their specified application rates. The permits should be read in conjunction with the relevant product label for information on withholding periods and other critical comments.
More detail on the permits is available from the information sheets found on the APVMA Portal. A direct link to each minor use permit PDF is provided in the tables below.
Note: New permits are regularly issued for fall armyworm control. Check the APVMA Portal for the most current information.
Table 1 Current permits in Australia for sorghum crops (as at 20 May 2020)
| APVMA permit | Insecticide | Rate of product/ha | IRAC/MOA classification |
| alpha-cypermethrin 100 g/L | 400 mL/ha | 3A | |
| methomyl 225 g/L | 2000 mL/ha + wetter; see label | 1A | |
| zeta-cypermethrin 100 g/L | 500 mL / ha | 3A | |
| alpha-cypermethrin 100 g/L | 220 - 280 mL/ha | 3A | |
| alpha-cypermethrin 250g/L | 88 – 112 mL/ha | 3A | |
| Spinetoram 120g/L | 250-300 mL/ha | 5 | |
| Gamma-cyhalothrin 150g/L | 60 mL/ha |
|
*Insecticide Resistance Action Committee ** Mode of Action
Important disclaimer
The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it.
Management in grains, canola and pulses -
wheat, barley, oats, canola, pulses
A variety of grain crops are grown in Western Australia across different regions. In the north, fall armyworm is expected to persist year round, therefore grains grown in those areas are likely to be vulnerable throughout the production period. The potential of fall armyworm to migrate into the southern grain producing areas of Western Australia, such as areas of the Wheatbelt and Great Southern, and damage crops at various growth stages is not yet known. Growers should be vigilant and aware of this new pest when monitoring cereal, canola and pulse crops for pests.
Cereals, canola and pulses are considered secondary host plant for fall armyworm and may not be attacked if a preferred host plant, such as sorghum or maize, is available. Information about fall armyworm in cereals, canola and pulses can be found below.
Information on the management of fall armyworm on sorghum and maize and sweet corn crops is available on separate webpages.
Cereals, canola and pulses are considered secondary host plants and may not be attacked if a preferred host plant, such as sorghum or maize, is available. Information about fall armyworm in cereals, canola and pulses can be found below.
Damage
Cereals (wheat, barley, oats)
Kansas State University advises:
- The first sign of damage is “window-pane” injury caused by tiny larvae chewing on seedling leaves.
- The larvae, which are usually too small to be easily observed at this time, hide in or around the base of seedlings.
- Within a few days, the larvae become large enough to destroy entire leaves.
- Larvae increase in size at an exponential rate, and so do their food requirements.
- Later instars do the most damage, sometimes destroying entire stands, and are the least susceptible to insecticides.
- Without treatment, problems can continue until larvae reach maturity or until a frost kills caterpillars.
Canola and pulses (chickpea, faba bean, field pea, lentil, lupin, mungbean)
For seedlings, tiny larvae produce small window-pane damage to leaves, while larger larvae can cut the base of the plant. Mature plants suffer attack on reproductive parts. Later larval instars make larger holes, causing ragged leaf damage, and produce larger droppings.
Monitoring
Until we acquire a greater understanding of fall armyworm in southern grain growing regions of Western Australia, it is recommended that growers monitor crops for fall armyworm in a similar way as currently conducted for existing caterpillar pests which are similar in size and cause comparable damage to vegetative and reproductive parts of plants.
Cereals (wheat, barley, oats)
Current monitoring techniques for existing armyworms include visual inspection for leaf damage and caterpillars at early growth stages and a combination of visual inspection and sweep netting for later growth stages. Thresholds are not well-established for existing armyworms in Western Australia.
For information on the commonly occurring armyworm in cereal crops, see DPIRD’s webpages Diagnosing armyworm and Armyworm: economic considerations for management.
Fall armyworm is most active in the morning or late afternoon and may hide during other times of the day.
Canola
Crops from seedling stage through to bolting should be inspected for leaf-chewing damage caused by caterpillars. Cutworms and weevils may also cause chewing damage.
To monitor fall armyworm in flowering or maturing canola in spring, fall armyworm should be identified and counted via sweep netting programs, which are already occurring for native budworm and diamondback moth caterpillars.
Kansas State University advises:
- Examine plants along the field margin as well as in the interior because fall armyworm often moves in from road ditches and weedy areas.
- Look for windowpane damage in young canola plants or cut plants.
- Do not allow fall armyworms and cutworms to reduce plant numbers at the seedling stage.
Pulses (chickpea, faba bean, field pea, lentil, lupin, mungbean)
Crops from seedling stage through to bolting should be inspected for leaf chewing damage caused by caterpillars. Cutworms and weevils may also cause chewing damage.
To monitor fall armyworm in flowering or maturing pulse crops in spring, fall armyworm should be identified and counted in sweep netting programs, which are already occurring for native budworm caterpillars, which look very similar.
When to take action
Cereals (wheat, barley, oats)
Thresholds for fall armyworm in cereals are not well-established. The common armyworms, which are known to cause damage to cereal crops in Western Australia, especially maturing barley, may pose a greater risk than fall armyworm, however growers should be vigilant and monitor for fall armyworm in their crops.
Kansas State University advises that if 25-30% of wheat plants have feeding damage, watch closely and apply a control if plant stands are threatened.

Canola
Thresholds for fall armyworm in canola are not well-established. Applying the threshold calculations for native budworm may assist in providing optimal management of fall armyworm
Kansas State University advises:
- Economic thresholds are not well established in canola, but damage is usually minor and yield loss minimal if the plants are healthy and growing vigorously and populations are not excessive.
- Producers should watch fields closely and only treat if larval populations appear to threaten stands, cause significant defoliation or begin feeding on seed pods.
Management
An integrated pest management (IPM) approach should be considered for protecting crops from infestations of fall armyworm.
Fall armyworm has entered an existing suite of pests and associated natural enemies, systems of production and pest management programs. Careful consideration needs to be given to any actions taken for fall armyworm control that may have adverse effects on management options already in place for other pests in grains crops, especially where natural control agents are used.
Natural enemies of other insects in the same group as fall armyworms that are already present in grains crops may also attack fall armyworm. There is already evidence of wasps parasitising fall armyworms in maize and sorghum in Kununurra. However, the effect of natural enemies with fall armyworm will become more clear as our experience with the pest accumulates.
An important management practice is to maintain farm biosecurity measures and implement good farm hygiene, and remove alternative hosts such as weeds and volunteer crop plants, especially during periods, and in places, where fall armyworm would not easily be able to survive year-round.
Other cultural practices, such as trap cropping, may reduce fall armyworm numbers. Understanding the value of the various cultural practices for fall armyworm management that have been tested overseas will require further study under Australian conditions.
In the short term, insecticides are available to help protect crops from fall armyworm. However, this insect has a reputation for developing resistance to insecticides. Resistance management strategies will therefore be required to maintain the effectiveness of insecticides for controlling this pest.
Application of insecticide will be most effective if applied late in the day and into the night, when larvae become more active and emerge from protected areas of the plant.
Insecticides available for use against fall armyworm includes those available under recently approved Australian Pesticides and Veterinary Medicines Authority (APVMA) minor use permits. Also available for use in WA, are any insecticides registered for use on crops for control of other insects if those products are considered effective on fall armyworm and provided they are applied according to label details. See section 87 ‘Use in accordance with label’, page 53 of WA Health (Pesticides) Regulations 2011.
The following tables list the details of pesticides available with current permits in Australia for winter cereals (Table 1), canola (Table 2) and pulses (Table 3), and their specified application rates. Permits for other grains are also available. The permits should be read in conjunction with the relevant product label for information on withholding periods and other critical comments.
More detail on the permits is available from the information sheets found on the APVMA Portal. A direct link to each minor use permit PDF is provided in the tables below.
Note: New permits are regularly issued for fall armyworm control. Check the APVMA Portal for the most current information.
Table 1. List of current permits in Australia for winter cereals (as at 7 August 2024)
| APVMA permit | Insecticide | Rate of product/ha | IRAC* MOA** classification |
| Fawligen Spodoptera frugiperda nucleopolyhedrovirus (NPV) | 50 – 200 mL/ha | 31 | |
| Spodovir plus Spodoptera frugiperda nucleopolyhedrovirus (NPV) | 50 – 200 mL/ha | 31 | |
| Emamectin 17 g/L | 600 – 900 mL/ha | 6 | |
| Alpha-cypermethrin 250 g/L | 88 - 96 mL/ha | 3A | |
| Alpha-cypermethrin 100 g/L | 220 - 240 mL/ha | 3A |
*Insecticide Resistance Action Committee **Mode of Action
Table 2. List of current permits in Australia for canola (as at 7 August 2024)
| APVMA permit | Insecticide | Rate of product/ha | IRAC* MOA** classification |
| PER 90820 | Fawligen Spodoptera frugiperda nucleopolyhedrovirus (NPV) | 50 – 200 mL/ha | 31 |
| PER 91477 | Spodovir plus Spodoptera frugiperda nucleopolyhedrovirus (NPV) | 50 – 200 mL/ha | 31 |
*Insecticide Resistance Action Committee **Mode of Action
Table 3. List of current permits in Australia for pulses (refer to permits for pulse types) (as at 7 August 2024)
| APVMA permit | Insecticide | Rate of product/ha | IRAC* MOA** classification |
| Fawligen Spodoptera frugiperda nucleopolyhedrovirus (NPV) | 50 – 200 mL/ha | 31 | |
| PER 91477 | Spodovir plus Spodoptera frugiperda nucleopolyhedrovirus (NPV) | 50 – 200 mL/ha | 31 |
| Alpha-cypermethrin 100 g/L | 220 - 280 mL/ha | 3A | |
| Alpha-cypermethrin 250 g/L | 88 - 112 mL/ha | 3A |
*Insecticide Resistance Action Committee **Mode of Action
For a list of registered insecticides for cereals, canola and pulse crops in Western Australia, including for other armyworms in cereal crops, see DPIRD PestFacts WA team’s Autumn winter insecticide guide and Winter spring insecticide guide.
For more information on fall armyworm in grain crops visit:
Important disclaimer
The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it.
Management in horticulture - vegetables and fruit
Damage
Grass type crops are the most commonly damaged by fall armyworm but the range of horticultural crops reported as hosts is extensive and includes fruit and nut crops, vegetables, various herbs, floriculture and turf. Whether fall armyworm causes damage to any horticultural crop may be a result of seasonal abundance or proximity to more preferred host plants. The pest status of fall armyworm on horticultural crops in Western Australia will become more clear with time.
Among vegetable crops in the south-eastern states of the United States, only sweet corn is regularly damaged. For information about management of fall armyworm in sweet corn, please see the Management in maize and sweet corn page.
In some years, fall armyworm is a major pest of tomatoes and capsicums in south-eastern United States. Fruit can be attacked, leading to premature drop and fruit rot. Fall armyworm is also encountered as a quarantine pest in capsicums exported from the United States to Europe. Other vegetables affected by fall armyworm include crucifer crops, melons and sweet potatoes.

The young larvae first feed on leaves near the ground where the damage may go unnoticed. Leaf damage by young larvae appears in the form of ‘windowing’ where only the epidermis remains. By the second or third instar, larvae begin to make holes in leaves, and eat along leaf edges. Older larvae cause extensive defoliation, often leaving the plant with a ragged, torn appearance. The number of larvae are usually reduced due to cannibalistic behaviour.
Because horticultural crops are used for human consumption, there are generally low damage thresholds for produce or pest presence. This market requirement demands regular monitoring to protect crops from the adverse effects of fall armyworm.
Monitoring
If fall armyworm becomes an important or consistent pest in a particular region, a pheromone trap can be deployed to check on the presence of moths as a warning of a potential pest situation.
Early detection is essential. Young seedlings in trays should be checked for egg masses and larvae before they are transplanted in the field. Regularly check crops in the field and record the presence of egg masses and larvae that may be recognised by their characteristic damage to leaves – “windowing” indicates the start of an infestation and gross leaf damage points to the presence of older larvae. Regular, timed searches across crops will ensure the start of an infestation or hot spots early are not missed.
Be sure to clearly identify fall armyworm where damage is thought to be caused by the pest.
When to take action
No action thresholds are available for the range of horticultural crops listed as hosts of fall armyworm. Experience with similar pests, such as cutworms, armyworms and budworms (Helicoverpa, heliothis), will help decide whether infestation levels of fall armyworm require intervention.
Management
An integrated pest management (IPM) approach should be considered for protecting horticultural crops from infestations of fall armyworm.
Fall armyworm has entered an existing suite of pests and associated natural enemies, systems of production and pest management programs. Careful consideration needs to be given to any actions taken for fall armyworm control that may have adverse effects on management options already in place for other pests of horticultural crops, especially where natural control agents are used.
Natural enemies of other insects in the same group as fall armyworms are already present in many horticultural crops and these may also attack fall armyworm. There is already evidence of wasps parasitising fall armyworms in Kununurra. However, the effect of natural enemies with fall armyworm will become clearer as our experience with the pest accumulates.
An important management practice is to maintain farm biosecurity measures and implement good farm hygiene, and to remove alternative hosts such as weeds and volunteer crop plants, especially during periods, and in places, where fall armyworm would not easily be able to survive year-round.
Other cultural practices, such as trap cropping, may reduce fall armyworm numbers. Planting maize or sorghum as an attractive trap crop has led to reductions of fall armyworm in horticultural crops. Understanding the value of the various cultural practices for fall armyworm management that have been tested overseas will require further study under Australian conditions.
In the Americas, Spodoptera frugiperda nuclear polyhedrosis virus (SfNPV) has been successful in significantly reducing the damage caused to maize crops, but this product is now available in Australia and can infect and kill young fall armworm larvae. The NPV that controls Helicoverpa larvae is not considered to be effective against fall armyworm.
Insecticides that contain the bacterium Bacillus thuringiensis (Bt) would most likely kill larvae of fall armyworm, especially if applied when larvae are small. However, considering they need to be ingested and larvae feed in concealed areas, foliar applications of Bt products are recommended with caution for use in horticultural crops.
In the short term, insecticides will be relied upon to protect crops from fall armyworm. This insect has a reputation for developing resistance to insecticides. Resistance management strategies will be required to maintain effectiveness of insecticides for controlling fall armyworm.
Application of insecticide will be most effective if applied late in the day and into the night, when the larvae become more active and emerge from protected areas of the plant.
Insecticides available for use against fall armyworms include those available under recently approved APVMA minor use permits. There are numerous permits for horticultural crops. Where required, APVMA permits should be read in conjunction with the relevant product label for information on withholding periods and other critical comments.
Also available for use in WA, are any insecticides registered for use on crops for control of other insects if those products are considered effective on fall armyworm and provided they are applied according to label details. See section 87 ‘Use in accordance with label’, page 53 of Western Australia’s Health (Pesticides) Regulations 2011.
More detail on the permits is available from the information sheets found on the APVMA Portal. A direct link to each minor use permit PDF is provided in the tables below.
Note: New permits are regularly issued for fall armyworm control. Check the APVMA Portal for the most current information.
Important disclaimer
The Chief Executive Officer of the Department of Primary Industries and Regional Development and the State of Western Australia accept no liability whatsoever by reason of negligence or otherwise arising from the use or release of this information or any part of it.
Webinars and other resources
To communicate current information about fall armyworm with industry in Western Australia, the Department of Primary Industries and Regional Development (DPIRD) will share information with industry by webinar.
A webinar was conducted for growers, agronomists and industry representatives in WA on 9 April 2020.
In coming months, DPIRD will conduct further webinars to share the latest information, as it evolves.
Webinar - Fall armyworm in Western Australia, 9 April 2020
- Presentation: Fall armyworm in Western Australia, Introduction, Dr Helen Spafford, Senior Research Scientist, DPIRD, and PowerPoint presentation
- Presentation: Fall armyworm surveillance, David Cousins, Senior Biosecurity Officer, DPIRD, and PowerPoint presentation
- Presentation: How to identify fall armyworm, Dr Melinda Moir, Senior Laboratory Scientist, DPIRD, and PowerPoint presentation
- Presentation: Management options for fall armyworm, Dr Helen Spafford, Senior Research Scientist, DPIRD, and PowerPoint presentation
- Questions and Answers (Q&As)
Webinar - Fall armyworm in Carnarvon, Western Australia, 6 May 2020
- Presentation: Fall armyworm in Carnarvon, Western Australia, Dr Helen Spafford, Senior Research Scientist, DPIRD PowerPoint presentation.
Fall armyworm Industry Updates
- Fall armyworm Industry Update #3, 30 April 2020
- Fall armyworm Industry Update #4, 28 July 2020
- Fall armyworm Industry Update #5, 27 August 2020
- Fall armyworm Industry Update #6, 12 November 2020
- Fall armyworm Industry Update #7, 11 February 2021
- Fall armyworm Industry Update #8, 5 July 2021
Media statements
- No further genetic resistance to fall armyworm, 2 July 2021
- Fall armyworm detected in Geraldton, 28 July 2020
- Fall armyworm detected in Carnarvon, 30 April 2020
- Fall armyworm found near Broome, 20 April 2020
- Invasive fall armyworm detected in northern Western Australia, 2 April 2020
- Horticulture growers urged to monitor crops for voracious pest, 11 February 2021
- GRAINS RESEARCH UPDATES 2021: Vigilance required to protect crops from new pests, 23 February 2021