Protecting oats - the super break (fast) crop
Oats are a high yielding break crop enabling growers to improve their response to changing seasonal conditions and price risks. They are inherently less prone to yield losses brought about through waterlogging and frosts. Oats are able to adapt well to early sowing windows and this reduces the risks of spring drought. Oats provide a competitive crop canopy and aid the management of weeds in the rotation, providing opportunities in harvest weed seed management for grain and in hay. There are further benefits gleaned from leaf disease management.
The WA industry is consistently producing half a million tonnes of oats per year, and there is significant potential to increase exports to nearby countries. More details can be found in the GRDC Research Updates 2017 paper titled "Future Opportunities for the Western Australian oat industry," prepared by Rob Loughman, Principal Scientist at the Department of Primary Industries and Regional Development.
Oat breeding and disease resistance in WA
The WA Oat Breeding Program is led by Pip Payne at the Department of Primary Industries and Rural Development, DPIRD (formerly Department of Agriculture and Food WA). It is part of the National Oat Breeding Program in partnership with SARDI (South Australian Research and Development Institute), funded by GRDC (Grains Research and Development Corporation) and RIRDC (Rural Industries Research and Development Corporation).
New lines undergo at least 5 years evaluation in trials across the agricultural area before being considered for bulk up and release. Disease assessments are carried out at nurseries in Carnarvon for Stem rust and Manjimup for Leaf rust, Septoria and Barley yellow dwarf virus (BYDV). Assessments for Bacterial Blight (Stripe blight & Halo blight), Cereal Cyst Nematode (CCN) and Red leather leaf (not found in WA) are carried out in South Australia.
Potential new varieties developed by the National Oat Breeding Program include:
- 03198-18, potentially a new grain variety with similar maturity to Mitika and is being bulked for release in 2018.
- 03126-35 is an early-mid season maturing line with potential as a milling grain which is also being bulked for a potential release in 2018.
- 06204-16 is another early-mid season potential milling oat with an expected release in 2019.
- 05096-32 is mid tall potential hay variety with early-mid season maturity. It combines improved septoria resistance with good rust and bacterial blight resistance. It is also being bulked for potential release in 2018.
| Variety | Stem rust | Leaf rust | BYDV | Septoria | Bacterial blight | CCN Resistance | CCN Tolerance | Red leather leaf |
| Bannister | R-MR | R | MS | S | MR-S | VS | I | MS |
| Carrolup (tall) | MS | S | MS | S-VS | MR-S | S | I | S |
| Durack (tall) | MR-MS | R-S | MS-S | S-VS | MR-S | R | MI/MT | MS |
| Kojonup | R-MS | S | MS | S-VS | MS-S | VS | I | MS |
| Mitika | MR-S | R | S | S-VS | MR | VS | I | S |
| Wandering | MS | VS | MR-MS | S-VS | MR-S | VS | I | MS |
| Williams (tall) | MR | R | MR-MS | MS | R | S | I | MS |
| Yallara (tall) | MR-MS | R | MR-S | MS-S | MR-MS | R | I | MS |
| 03198-18 | S | R | MS | S | MR | VS | - | MS |
| 03216-35 | MR | MR | MR-MS | MR-S | MR | R | MT | MR-MS |
| 06204-16 | MS | R | MR-S | MS-S | MS | S | - | - |
| 05096-32 | R | R-MR | MS-S | MR | MR | S | - | - |
Note: Stem rust, Leaf rust, BYDV and Septoria resistances are from WA trials. Bacterial blight, CCN and Red leather leaf reactions are from SA trials. Rust and BYDV reactions may vary in in different regions and with different seasonal conditions depending on the prevalent pathotype/serotype. Crop monitoring is essential. CCN resistance: a resistant variety retards nematode development, leading to lower nematode levels in the soil for subsequent crops.
Disease resistance abbreviations:
VS – very susceptible MR – moderately resistant R - resistant
S – susceptible MI – moderately intolerant T – tolerant
MS – moderately susceptible MT – moderately tolerant I - Intolerant
Oats more competitive than wheat and barley
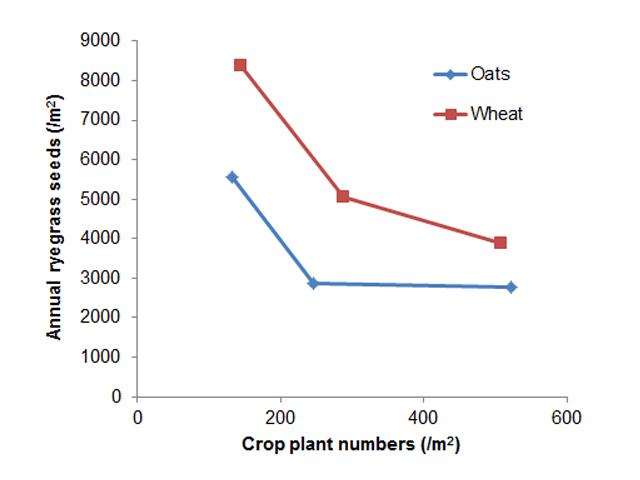
Oats are more aggressive against weeds than most other crops, certainly more so that the other cereal crops. For many years oats have been seen as the crop that growers can rotate to, to aid them in their management of weeds and weed related issues like herbicide resistance.
It is now legal to use Trifluralin in oats
While oats are competitive against weeds they are still subject to losses in yield and quality as a result of weed competition. Export hay has rigid quality standards, which include less than 5% foreign weed material such as annual ryegrass. There is also a nil tolerance to annual ryegrass toxicity (ARGT) and prickly weeds such as doublegee (Emex australis).
Managing weeds in oat crops recently became easier following the approval of a permit for the use of trifluralin on oats. The Australian Pesticide and Veterinary Medicines Authority (APVMA) approved the permit in April 2017 (Permit Number 84040). According to the permit, trifluralin 480 (any registered product) at 2 L/ha incorporated by seeding, can be used for suppression/control of certain annual grasses and broadleaf weeds in both oaten hay and milling grain oats.
The approval of the permit was a combined effort between the Grain Industry Association of Western Australia (GIWA) who progressed the permit through the APVMA, GRDC through the Pathways to Registration project and DPIRD who supplied the majority of the research data.
Traditionally trifluralin has been damaging to oats under a full cut out seeding systems. Widespread adoption of no-till seeding systems coupled with optimum seeding depth, have made it safer to use trifluralin on oats. This seeding system separates crop seeds from the zone of herbicide treated soil. Herbicides registered on oats such as metolachlor + diuron, terbuthylazine and chlorsulfuron at label rates; provide only suppression of ryegrass and other grass weeds.
Dr Harmohinder Dhammu has spent the past 10 years researching the impact of herbicides including trifluralin on the tolerance of a range of crop varieties. This permit is primarily based upon his research work and the work of others at DAFWA over the past 20 years conducted with support from GRDC.
The permit is valid from 4th April 2017 to 31st March 2022 (5 years) in WA only and is held by GIWA. Further research work on interaction of trifluralin with new oat varieties and seeding speed continues under a GRDC funded project (DAW00227) which will assist in registration of this herbicide on oats in future.
However, if not used correctly, trifluralin can still cause damage to oats. It is suggested that oat growers follow the permit restrictions, usage instructions and withholding periods for trifluralin. The following critical points need to be considered when using trifluralin on oats:
- Use trifluralin with knife/blade points and press wheels only. Press wheels prevent dragging treated soil back into the seed furrow.
- DO NOT use trifluralin with disc openers or similar,planting equipment.
- While seeding oats, maintain slow to moderate speed to avoid throwing treated soil into adjacent sowing furrows. This is especially critical at higher trifluralin use rates.
- Avoid sites that waterlog or where furrow walls may collapse, as crop establishment and vigour may be reduced.
- Treated soil movement into furrows due to rain or wind may cause crop damage.
- Use the highest trifluralin rate along with a higher water volume where there is stubble coverage. Stubble coverage can reduce weed control below acceptable levels.
- Oats should be seeded at the optimum seeding depth (3-4 cm), as trifluralin selectivity is based upon differing positions of weed and crop growing points. Shallow sowing increases the risk of crop damage.
- For best results apply trifluralin as close as possible to sowing.
- Trifluralin is volatile and disappears from exposed surfaces. Loss, of effective herbicide, is hastened by high temperatures, winds or warm moist soil.
- Observe 75 days withholding period for trifluralin for grazing oats or cutting oats for stock feed and hay.
- For improved and broad-spectrum weed control, trifluralin 480 2 L/ha can be applied mixed with Terbyne® Xtreme® up to 1.2 kg/ha.
- A range of oat varieties (Bannister, Carolup, Durack, Hotham, Kojonup, Mitika, Yallara, Wandering and Williams) have shown good tolerance to trifluralin under knife point and press wheel seeding systems in herbicide tolerance trials in WA. It is expected that non-tested oat varieties with a similar genetic background (to the tested ones) would possess similar tolerance to trifluralin.

[PDF]APVMA PERMIT 84040 OATS Treflan 480 Herbicide + Trifluralin 480
Grains Industry Association of Western Australia
Are oats susceptible to root lesion nematodes?

There are two main species of root lesion nematodes (RLN) which impact on broadacre crop production in WA, Pratylenchus neglectus and P. quasitereoides (formerly P. teres). It is estimated that at least 70% of cropping paddocks are infested with RLN’s and that in 50% of these paddocks RLN’s are yield limiting. Root damage caused by RLN’s cause yield losses in wheat and barley. Losses of at least 5% (and more often 10–15%) are common for wheat and barley and are estimated to cost approximately $190 million p.a. in Southern and Western Australia.
Currently, there are no economically viable chemicals available to control RLNs in broadacre cropping. Management choices are based on crop rotation and variety selection.
Traditionally its been thought that oats are the most resistant of our cereal crops but RLN has been identified in an increasing number oat crops after growers and their consultants noticed RLN symptoms. As oats cropping increases we are regularly asked by growers and consultants
(a) whether oats are susceptible to RLN’s and
(b) if they have a paddock which is infested with an RLN species, will oats exacerbate the problem?
Carla Wilkinson and Sarah Collins (DPIRD) are currently undertaking a two year COGGO supported project to try to answer these questions (2017-2018).
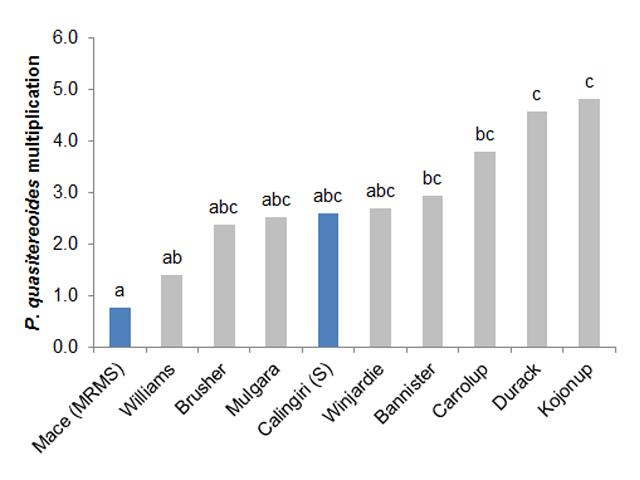
Preliminary data have shown that the most commonly grown milling and hay oat varieties are as susceptible as the most susceptible wheat, Calingiri, to both P. neglectus and P. quasitereoides.
Soil (with roots if present) was taken from the plots at the beginning and the end of season to determine whether the RLN species of interest increased under the different wheat (2) and oat (8) varieties. If a crop is resistant then RLN will not increase over the season. Both species of RLN increased under all of the oat varieties over the season. In the trial testing P. quasitereoides there was a difference between varieties (Figure 2). For P. neglectus there was no difference in multiplication between the varieties tested with all varieties supporting a multiplication by a factor of 4 over the season.
Oats are susceptible to a number of leaf diseases
Oats are susceptible to a number of foliar fungal rust and blotching diseases, with stem rust, leaf rust and septoria probably the big three fungal diseases and bacterial blight caused by a bacterial pathogen (as the name suggests) also being important in some areas.
Within the crop protection group at DPIRD, Manisha Shankar conducts stem rust screening trials at Carnarvon in collaboration with breeders. This location is ideal as it is removed from the major oat production zones and provides the warm moist conditions that favour the disease. The DPIRD cereal pathology group also provides advice for the leaf rust screening trials at Manjimup.
Stem rust and leaf rust are always going to be a challenge for our oat crops as both diseases are hosted by wild oats, an abundant and widespread weed. Varieties do differ in their susceptibility to each of these foliar diseases which may vary dependent on the pathotype present.
Septoria is a stubble borne disease of oats and varieties differ for their susceptibility to this disease. Septoria can impact green leaf which in hay crops will influence hay quality and in grain crops can reduce yield or impact grain size and quality. Grain staining in varieties such as Bannister can seriously impact on delivery quality this staining is related to septoria infection (and weather conditions).
Recent trial work conducted by Ashton Gray and Garren Knell of ConsultAg (with collaboration from Geoff Thomas DPIRD) determined that foliar fungicide can reduce the severity of Septoria blotch in Bannister oats. Fungicide application at or around flag leaf emergence seemed the most effective for disease control and yield response. Yield responses of up to 600kg/ha above the 4.1 t/ha untreated yield were achieved in the high yielding environment.
Multiple applications of fungicide can add benefit under high disease pressure, with the first application at stem extension allowing some flexibility in the timing of the second application. Treatments which reduced disease severity in the canopy had greatest impact on grain staining, fungicide applied at heading was ineffective without prior applications.
Growers in medium to high yielding environments should consider a two spray strategy if growing Bannister oats (or other susceptible varieties) on oat stubble.
Seasonal conditions from grain fill to harvest have a significant impact on fungal grain staining but applying late fungicides to reduce grain staining may be an unreliable method to reduce overall staining on seed.
An economic return of up to $215/ha was achieved with the improved yield and grain quality achieved from a double fungicide spray at the commencement of stem elongation and flag leaf emergence.
Crown rot and oats

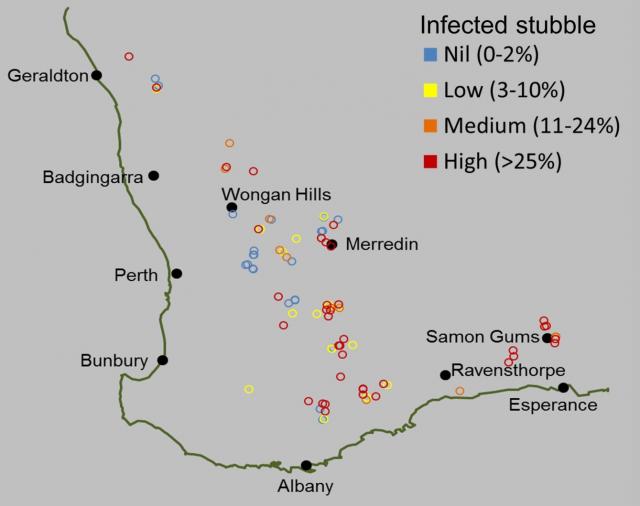
Fusarium crown rot, caused predominantly by the stubble-borne fungus F. pseudograminearum, is one of the major root and crown disease constraints on cereal production in Australia. In 2009, it was estimated to cost Australian grain growers $97 million annually in wheat and barley (Murray and Brennan, 2009, 2010). WA’s losses to this disease were estimated at that time to be $7 million annually. In 2014, many growing regions in WA were impacted by crown rot, exacerbated by dry weather conditions during grain fill. For example, reports by agronomists and growers from Merredin indicated there were 30-50% losses in wheat paddocks infected with crown rot (refer to 2014 increase in crown rot in wheatbelt for more information).
A two-year stubble survey in 2013 and 2014 by the DAFWA (now DPIRD) found crown rot was present in most growing regions at levels potentially damaging to yield (figure 3). In a few southern sites (e.g. Borden, Jerramungup and Esperance) from this survey, F. culmorum, which can also cause crown rot, was also confirmed.
Relatively little is known about the impact of crown rot on oats. A project commenced in 2016, as part of the Grains Flagship’s supported by DPIRD, to develop susceptibility ratings for oat varieties. Department research officer Daniel Hüberli is leading this project.
Trials in 2016 at a low rainfall site in Merredin and a high rainfall location near Pingelly showed some varieties suffered greater yield loss from crown rot of between 5 and 8% than others with minimal or no losses (Figure 4). The trials have been replicated this year at Merredin and a higher rainfall site at Muresk, near Northam. Preliminary results indicate that oats in general are more tolerant to crown rot than wheat.
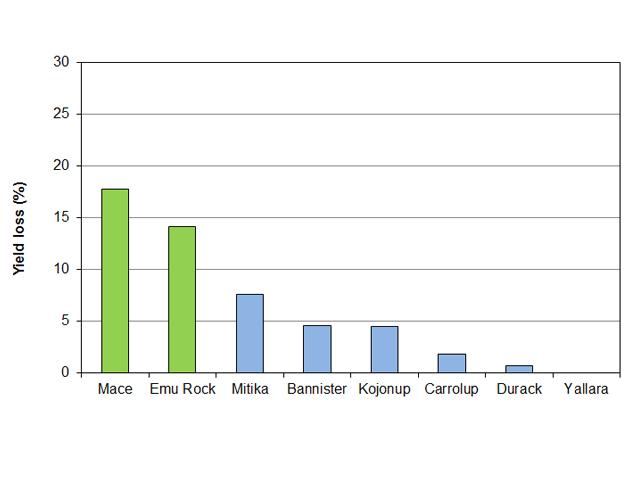
As in 2016, six varieties are being tested in each trial, including Bannister, Carrolup, Durack, Kojonup, Mitika, Williams and Yallara, alongside two wheat varieties – Mace (susceptible (S) to crown rot) and Emu Rock (moderately susceptible (MS) to crown rot). The trials consist of three paired plots with four replicates including two inoculated with F. pseudograminearum or F. culmorum and a nil plot.
In addition, all plots at the 2016 oat trials at Merredin and Pingelly were soil sampled to test the amount of crown rot inoculum present using PreDicta B to ascertain whether there has been an increase or decrease in the inoculum generated by the different oat varieties. Preliminary data suggests that both oat and wheat increase F. pseudograminearum, but that oats increase F. culmorum more than wheat (Figure 5). This trend was also observed at Merredin. Both trials have been over-sown this year with wheat and we will be monitoring the level of disease generated under the different oat varieties.

The research will include a survey of the oat National Variety trials so trial findings can be extended to other regions. This complements the department’s work with the National Crown Rot project DAN00175 (focusing on wheat and barley), funded by the Grains Research and Development Corporation. All of the results are intended to support future Variety Sowing Guides for oats and to assist the development of varieties with improved disease resistance.

Growing season resource 2017
This growing season is likely to be a challenging season for many agricultural businesses across the grainbelt.
Department officers from across the grainbelt are liaising with local grower groups, consultants and farming organisations to ensure agribusinesses receive the support they require.
A Season 2017 webpage is available on the department’s website that harnesses a vast range of information to assist landholders to navigate the season ahead.
It includes useful information on climate and weather tools, crop agronomy, livestock management, farm budgeting and health and financial information.
This website will be updated over coming months to provide timely and relevant advice, tools and networks as the season progresses.
Contacts
Oat Breeding in WA
Pip Payne
Research Officer
phillipa.payne@agric.wa.gov.au
Ph: +61 (0)8 9690 2146
Trifluralin and Herbicide tolerance
Harmohinder Dhammu
Research officer
harmohinder.dhammu@agric.wa.gov.au
Ph: +61 (0)8 9690 2217
Root Lesion Nematodes
Carla Wilkinson
Research officer
Carla.wilkinson@agric.wa.gov.au
Ph: +61(0)8 9368 3862
Sarah Collins
Research officer
Sarah.collins@agric.wa.gov.au
m: 0404 488 113
Leaf diseases
Manisha Shankar
Snr research officer
Manish.shankar@agric.wa.gov.au
Ph: +61(0)8 93683533
Geoff Thomas
Research officer
Geoff.j.thomas@agric.wa.gov.au
M: 0428 947 287
Crown Rot
Daniel Huberli
Research officer
Daniel.huberli@agric.wa.gov.au
M: 0427 426 522
References
Murray GM, Brennan JP (2009). Australasian Plant Pathology 38: 558-570.
Murray GM, Brennan JP (2010). Australasian Plant Pathology 39: 85-96.