Aphids – WA’s insect problem children
Aphids are tiny soft-bodied insects from the family Aphididea. They are usually pear shaped and approximately 1-6 mm long.
Why are aphids are a problem to the WA grains industry?
Feeding damage
Aphids can cause feeding damage to grain crops. Both adult and nymph aphids can suck sap causing direct feeding damage to crops from seedlings to grain fill. The feeding of large populations can lead to seedling death or stunting, tiller or flower abortion and reduced seed set and size, ultimately limiting grain yield. The degree of damage depends on the percentage of tillers or plants infested, the number of aphids per tiller or branch and the duration of the infestation.
Vectors for transmitting plant diseases, particularly viruses
For example, oat aphids and corn aphids can transmit Barley yellow dwarf virus in cereal crops reducing yields by up to 10%.
Toxins
Russian wheat aphid (RWA) can inject a toxin into wheat and barley crops and have caused yield losses of up to 80%. Symptoms of RWA damage include streaking, leaf curling and redness in both wheat and barley crops.
They have been detected in South Australia, Victoria, New South Wales and Tasmania but not WA as yet. Growers and consultants are asked to report any findings, either absence or presence of RWA in 2017 via the MyPestGuide and PestFax reporting apps.
SA and NSW consultants claim that predators and aphid-killing fungi can reduce populations and there are emergency use permits for chlorpyrifos (500g/L @ 600mL/ha) and pirimicarb (500g/L @ 125-250g/ha).
Resistance to chemicals
Of Australia’s aphid pests of cereals, canola and pulses, the green peach aphid (GPA) is the only aphid, so far, to have developed insecticide resistance. GPA has been found to be resistant to organophosphate, carbamate and synthetic pyrethroid insecticides.
Reproduction and rapid spread
Aphids can reproduce and spread rapidly. In WA, aphids are female and can give birth to live young without the need to mate. While they are generally wingless, they can develop wings to allow them to migrate to new areas for colonisation
Expected aphid activity this season
Recent climate models are forecasting below average rainfall and warmer daytime temperatures this growing season, and these conditions are ideal for aphid reproduction. Aphids will consume more plant juices in warmer temperatures and inflict more damage when crops are young. Water stressed plants are also more susceptible to aphid damage.
DAFWA conducts research into aphid identification, monitoring, and spray thresholds
Aphid infestations can result in economic losses in crops. This however is not always the case, so time, effort and money can be saved by:
- proper identification,
- monitoring and
- applying thresholds which are the point at which spraying is economically worthwhile to prevent yield or quality limitations.
We are focusing our research on the main grain crops produced in WA which are cereals (including wheat, barley and oats), canola, and pulses (including lupins and field peas).
Identifying aphids
Once you have found your aphids and determined that they are at a level requiring some sort of intervention it is vital that you identify the type of aphid you have. Take the time to identify the aphids correctly as this will make a difference to the type of control you employ or if control is warranted at all.
Use visual inspection as a first step. Aphids are specific to particular host crop types, for example oat and corn aphids infest cereal crops, but are a never pest on pulses or canola and cabbage aphids will be found on canola but will never be a pest on non-brassica hosts. Where and when the aphids prefer to feed and congregate will also provide information into which species of aphid you are dealing with.
Aphids trapped in the air (winged aphids) can also be identified using a slide mount technique and relying on physical features and taxonomic keys. This can be very time consuming and difficult if there are many specimens to go through. Identification can be made more difficult as there is no host to directly connect with the specimen and winged aphids can look very different than the wingless versions.
DNA techniques are becoming more popular for identification of aphid species. The DAFWA diagnostics laboratory is able to identify some of the common aphids by sequencing the CO1 gene of the aphid. In due course it is planned this technique could be applied in the field using in-field molecular diagnostics such as LAMP. See section below on how Benjamin Congdon is using LAMP to detect virus infection in aphids.
Canola aphids
There are 3 types of aphids that attack canola crops - turnip aphids (TA), cabbage aphids (CA) and green peach aphids (GPA). Often one or more are seen on the same plants together. Turnip and cabbage aphids are generally the two aphid species that mostly damage Australian canola crops, while GPA does not generally do much damage except with moisture-stressed seedlings.
Information on how to diagnose canola aphids can be found through this link to the MyCrop pages.
GPA is often found feeding on the undersides of canola leaves in established flowering crops where they do little to no feeding damage. Yield loss in canola can be caused by GPA transmitting Beet western yellows virus (BWYV; syn Turnip yellows virus, TuYV) to canola plants prior to flowering.
A 2016 trial in Geraldton led by DAFWA Research Officer Svetlana Micic determined if GPA feeding caused yield loss in canola in the absence of the virus. Insect exclusion tents were used and GPA were introduced at 3 separate stages; early (May 27), mid (June 21) and late (July 19). GPA populations were then monitored 3 times throughout the season, at 98, 111 and 128 days after sowing and allowed to persist through to harvest with no insecticide applied. This work was funded as part of DAFWA’s Boosting Grains Research and Development Flagship.
Despite the high levels of GPA established, yield loss from GPA feeding damage to flowering canola was minimal with no impact on plant growth or yield. Svetlana's 2017 GRDC Research Update update paper titled "Minimal yield loss of canola to green peach aphid" can be viewed on the DAFWA website.
In another trial at Geraldton (2015) funded by Council of Grain Grower Organisations (COGGO), DAFWA entomologist Svetlana Micic recorded the numbers of cabbage aphid and GPA on a weekly basis. The aphid numbers were very high with almost 100% of plants infested. The species composition of the aphid population was approximately half GPA and half cabbage aphid at July 16 when leaf counts stopped. She concluded that the main cause of damage to the canola was from cabbage aphid and that the length of cabbage aphid colony on spikelets at flowering stage was directly proportional to yield loss. Seed quality, oil content and seed size was also affected by aphid colonisation.
This indicates that it is necessary to correctly identify the aphid species to ensure the right control measures are used. In most cases, it would not be economical to use insecticide to prevent feeding damage for GPA alone.
Cereal aphids
The most common aphids that infest cereal crops are the corn and oat aphids, with oat aphid generally being the most abundant species in cereal crops. Russian wheat aphid is not yet known to be present in WA. It is important to monitor crops to detect the incidence (or absence, probably more important) of this aphid species.
Diagnosing cereal aphids (MyCrop)
Diagnosing Russian wheat aphid (MyCrop)
Aphids of other grain crops
Lupins are most vulnerable to feeding damage from aphids during the budding and flowering stages. Severe damage from feeding can cause buds to drop, flowers to abort and reduce pod set. As in cereals, aphids produce honeydew on the plant surface which a sooty mould can grow on and further reduce plant health. In susceptible lupin crops, yield losses of up to 90% can be experienced by feeding damage if the aphids are not controlled. Varieties with intermediate resistance can lose up to 30% yield.
It is also important to note that in addition to feeding damage affecting yield, grain legumes can provide host for virus spread.
Diagnosing aphids in field peas
Monitoring crops for aphids
The presence of aphids in crops does not necessarily mean that they will cause a yield or quality loss. Knowing the level of aphids required to reach a stage where economic loss is probable (i.e. economic threshold), is important to both spray at the right time and prevent unnecessary sprays which are costly and leading to insecticide resistance from over-exposure. This threshold may be much lower at crop emergence/seedling establishment when transmission of viruses by aphids is a concern and preventative measures (e.g. seed dressings) may be required.
Monitoring crops for aphids is usually done by:
- Trapping winged aphids for early detection before they have colonized plants.
- Sampling plans which assess aphids directly from plants after they have flown in and colonized.
Aphid trapping for early detection: let’s make it automated
There are various types of traps used across agricultural industries to trap aphids such as suction traps which suck air at a height and collect aphids in a chamber and sticky traps which both attract aphids by their bright yellow colour and trap them using a sticky surface.
As part of DAFWA’s Boosting Grains Research and Development Flagship project, Development Officer Christiaan Valentine has designed automated aphid sticky traps which direct flying aphids to a yellow sticky card and send a high resolution image of the card three times per day via email. Aphids and other flying insects can then be identified and growers and consultants can have a heads up of when these aphid species are flying into crops, and this can be done without anyone having to physically be there to manually assess the trap. With the increasing size of farming properties, the physical monitoring of cropping areas is becoming more challenging, and early detection is especially important when transfer of a virus into crops, such as green peach aphids transmitting Beet western yellows virus into young canola crops, is a priority. While still in the design phase, the prototypes of these SMART traps have been tested and so far have been successful.
Sampling plans for aphids: let’s make it easier, quicker and targeted
As part of a GRDC-funded PhD project at UWA, DAFWA entomologist Dusty Severtson conducted a 3 year project determining how cabbage aphids move into a canola crop and what the best sampling method is to determine when and where to spray. With funding from DAFWA’s Boosting Grains Research and Development Flagship, a new App, CropScout, has since been developed to aid in the decision making process of whether to spray or not and whether sprays can be targeted to crop edges.
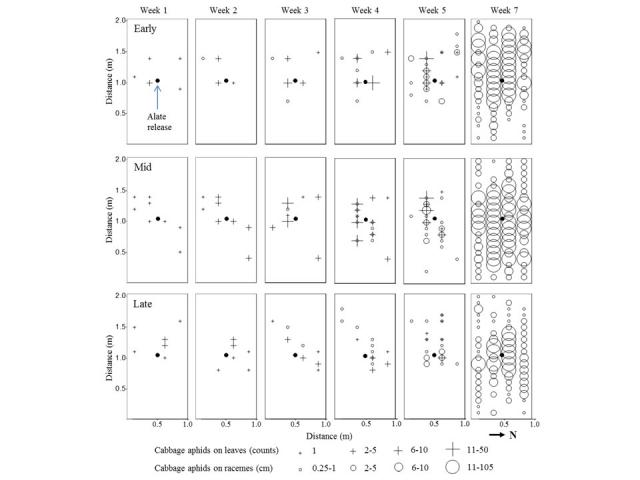
The research looked at both small plot trials to monitor aphid movement as well as large paddock scale to determine which sampling method would produce the most accurate results. The irrigated small canola plots were situated in tunnel houses at Shenton Park Field Station. There were three sowing times- early (10 May), mid (24 May) and late (7 June) with six replicates. Winged aphids were released into the middle of each plots and monitored to determine how they spread over the plots and the density of the infestation. As shown by the below images, the aphids colonised the leaves of nearest plants first, gradually expanding outwards and onto the flowering branches. From this research it was determined that cabbage aphids are often found only within 20m of the edges of a paddock. This spatial aggregation along edges is likely to occur with turnip aphids as well.
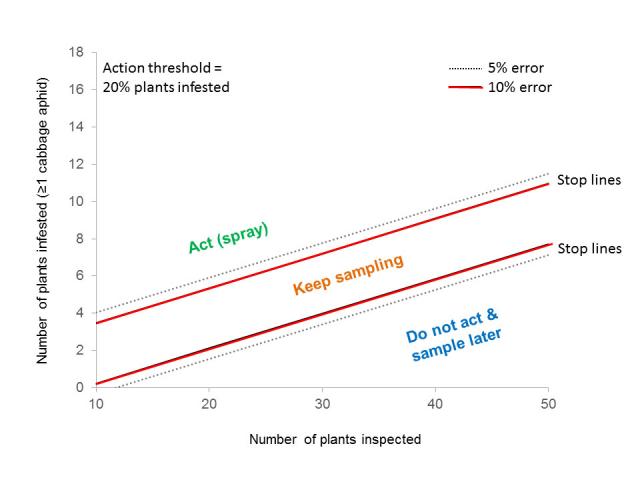
The current spray threshold for aphids in canola is 20% of plants infested on the branches. From large-scale aphid distribution data, Dusty developed a sequential sampling plan which reduced the number of plant inspections required to be confident that the number of plants infested with cabbage aphids is above or below this threshold. Sampling effort is also reduced by targeting plant inspections firstly to crop edges where the aphids are most likely to be found first. If above-threshold levels of aphids are found within 20 m of crop edge, then canola plants should be inspected further inwards. If none or below-threshold levels of aphids are found further inwards, then a decision to target insecticide sprays along the crop edge can be made. Sampling a crop in sections, known as ‘stratification’, reduces both the chances of overlooking areas of crop which may be above-threshold and the chances of applying insecticides to areas of crop which do not require it.
CropScout - coming soon
This improved method of sampling has recently been made into a smartphone application for iPhone and Android called “CropScout”. It will be part of DAFWA’s family of MyPestGuide applications and will be available for free download in the coming months ready for spring canola monitoring.
As seen in the images below, the sequential sampling algorithm runs automatically for the user as they inspect plants and click “yes” or “no” for whether a plant is infested with aphids or not. In addition to being able to assess crops relative to the aphid spray threshold, the app also harnesses the mobile phone’s ability to track GPS locations in and out of mobile range. Spray threshold results are then visualised on a map to see where exactly above-threshold levels of aphids are and whether insecticide sprays can be targeted (e.g. along a crop edge or portion of crop).
Aphid transmitted viruses
Many of the viruses found in WA grain crops are spread by aphids.
There are two main ways aphids can spread viruses and this impacts on the way virus diseases are managed:
- Persistently transmitted viruses. Once an aphid feeds on a virus-infected plant the aphid can spread the virus for its entire life. These viruses are found in the phloem of the plant and require the aphid to feed for long periods of acquire the virus. For this reason insecticides can be useful in managing virus spread as they kill the aphid prior to them spreading the virus further. Persistent viruses include Barley yellow dwarf virus (BYDV) and Beet western yellows virus (BWYV).
- Non-persistently transmitted viruses. These viruses are found throughout the whole infected plant. The aphid only needs to feed for a few seconds to acquire the virus and then spreads it when it feeds on the next plant. The aphid then loses the virus after feeding. Insecticides are not effective in controlling these viruses as they do not act quick enough to kill aphids prior them moving to new plants. Non-persistent viruses include Cucumber mosaic virus, Bean yellow mosaic virus and Pea seed-borne mosaic virus.
Viruses and aphids can be harboured in green bridge. In situations when there is substantial summer rainfall across the grainbelt the subsequent green bridge is likely to provide the ideal environment for aphid colonies to build up and move into new crops. This is how BYDV and BWYV enter crops as they are not seed-borne and need to survive between growing seasons in plant hosts to build up.
This season virus risk
Early rainfall this year indicated it was likely to be a high risk year for virus disease in crops across the grain belt, with aphid vectors building up in road side weeds (including wild radish). However, given the lack of follow up rainfall in April and May in many regions, when aphid population alight from weeds, there are few crops for them to move to. It is likely to be a low virus year in many regions.
Viruses spread by aphids
There are a number of virus diseases spread by aphids that can impact on crops grown in WA (Table below). This information is from green bridge and crops surveys, research projects (field trials) done by DAFWA over the last 25 years.
| Virus | Main crop affected (other crops) | Common vector | Management | Comments |
|---|---|---|---|---|
| Barley yellow dwarf virus (BYDV) and Cereal yellow dwarf virus (CYDV) | Wheat, barley, oats and grasses | Oat and corn aphid | Insecticide seed dressing in high risk areas | Not seed-borne. Early infection can cause yield losses |
| Beet western yellows virus (BWYV; syn Turnip yellows virus (TuYV) | Canola. (chickpea, faba bean, field pea, lucerne, medic and subterranean clover) | Green peach aphid | Insecticide seed dressing in high risk areas | Losses in canola can be up to 50% when crops infected pre-flowering |
| Pea seed borne mosaic virus (PSbMV) | Field peas (faba beans, lentil and chickpea) | Green peach aphid and cowpea aphid | Plant clean seed, use resistant varieties | Losses in field peas up to 25% |
| Bean yellow mosaic virus (BYMV) | Lupins (field peas, faba beans, lentil, chickpea sub. clover) | Green peach aphid and cowpea aphid | Control sub clover nearby lupin crops. | Early infection causes plant death, infection at podding causes Black pod syndrome. Up to 100% yield loss |
| Cucumber mosaic virus (CMV) | Lupins, (lentil, chickpea, sub. clover), | Green peach aphid and cowpea aphid | Plant clean seed | Seed-borne |
New technology for early warning of viruses
DNA techniques are becoming more popular for the detection of viruses in plants and also their aphid vectors. As part of DAFWA’s Boosting Grains Research and Development Flagship, Research Officer Benjamin Condon is using in-field molecular diagnostics (LAMP) to detect virus infection in aphids. Ben says they are developing the protocol to identify Turnip yellows virus (TuYV) in its green-peach aphid vector. Initial experiments have been highly successful with the protocol being able to detect one aphid carrying BWYV/TuYV in a batch of 99 healthy aphids, this shows how sensitive the test is. Current experiments are examining the best way to remove aphids from sticky traps and then test them with the LAMP machine. In-field validation of the techniques will be done this growing season with aphid specimens caught in traps in the grain belt and tested using the LAMP technology, as well as established molecular diagnostic tools such as PCR.
Identifying the virus in aphids found prior to the growing season and in winged aphids flying into emerging canola crops could provide an early warning of high BWYV/TuYV risk and help inform growers of the level of virus risk to emerging crops. This advanced field intelligence could inform decisions on seed dressings prior to sowing.
Understanding viruses to improve management
In the past few years, DAFWA has been involved in a number of other projects looking at viruses in grain crops including two PhD’s awarded to DAFWA staff in the last few years.
DAFWA Research Officer Ben Congdon completed his PhD in 2016 on Pea seed-borne mosaic virus (PSbMV) which is a serious disease of field pea crops. Within his PhD, Ben studied the relationship between the virus and the aphids and the extent of PSbMV infection in crops and seed stocks. He also looked into factors relating to its’ incidence, distribution, and possible control and developed a forecasting model to predict epidemics and optimise virus management decisions. For managing PSbMV sowing healthy seed is key. To avoid significant seed yield and quality losses, it is crucial to have seed-lots tested by DDLS to establish seed infection levels and only sowing if it is below the acceptable threshold recommended by the PSbMV SMS decision support system. If agronomically suitable, cv. Wharton offers comprehensive resistance to PSbMV.

DAFWA Research Officer Monica Kehoe studied the causal reasons for black pod syndrome (BPS) which can lead to devastating losses in narrow-leafed lupins. In her PhD, Monica found that while excessive vegetative growth and nutrient deficiencies were initially postulated, the cause of BPS is late infection with Bean yellow mosaic virus (BYMV). She recommends that BPS be managed through already established integrated disease management recommendations for BYMV in narrow-leafed lupins. Given the critical importance of the growth stage at the time of infection (i.e. late stage at podding) for BPS, the particular emphasis needs to be on maximising volunteer clover control within the crop. Equally important is avoiding planting next to or downwind of legume pastures, other narrow-leafed lupins crops, other grain legume crops or native legumes likely to contain sources of BYMV.
DAFWA Plant Virologists, Brenda Coutts and Monica Kehoe are currently involved in a GRDC national project ‘New tools and germplasm for Australian pulse and oilseeds breeding programs to respond to changing virus threats’. This project investigates lupin lines and varieties for reaction to Bean yellow mosaic virus and evaluates canola varieties for reaction to Turnip yellows virus (also known as BWYV) to determine if any virus resistance is present. From field evaluations of common canola varieties, none have been found to be tolerant or resistant to early BWYV infection. Current field and glasshouse trials will determine the yield and seed quality losses caused when canola is infected early (pre-flowering) or late (at mid-flowering). In addition, the usefulness of insecticide seed dressings is also being examined. This information will be useful in decision making for management of BWYV.
Brenda Coutts is also working on GRDC-funded national project “Effective control of Barley yellow dwarf virus (BYDV) in wheat”. In this project wheat germplasm is being screened for resistance/tolerance to BYDV infection, and this work is being done at Manjimup Research Station where BYDV and aphid vectors occur naturally. The germplasms being examined include many Chinese landraces and variety parental lines.
Contacts
Aphids
Dusty Severtson
Entomologist
dustin.severtson@agric.wa.gov.au
m: 0427 196 656
Svetlana Micic
Entomologist
svetlana.micic@agric.wa.gov.au
m: 0427 772 051
Viruses
Brenda Coutts
Virologist
brenda.coutts@agric.wa.gov.au
m: 0419 919 403
Ben Congdon
Virologist
benjamin.congdon@agric.wa.gov.au
Monica Kehoe
Virologist
monica.kehoe@agric.wa.gov.au