Perth Research Updates 2021 – Invertebrate topics covered by DPIRD staff

On 22 and 23 February 2021, approximately 600 growers, consultants and researchers attended the 2021 GRDC Grains Research Update Perth event.
The Perth event was followed by regional events at: Albany on 3 March, Merredin on 12 March, and Geraldton on 17 March. The Esperance zone Grains Research Update will be held in July, 2021 (details are yet to be confirmed).
A variety of research work was presented to the audience, with accompanying written papers. Presentation recordings and research papers are available on the GRDC Grains Research Update Perth web page.
This issue of 'Protecting WA Crops' will highlight the following invertebrate research papers authored or co-authored by DPIRD entomologists, Dr Dusty Severtson and Svetlana Micic:
- What is going on with fall armyworm and Russian wheat aphid in Western Australia?
- Controlling redlegged earth mites using intensive spring grazing
- Movement, breeding, baiting, and biocontrol of Mediterranean snails.
- Know what is in your chaff?
The research papers cover the topic of invertebrate crop protection. Other topics presented and published are available on the GRDC's Research Updates papers web page.
What is going on with fall armyworm and Russian wheat aphid in Western Australia?
Fall armyworm
Fall armyworm (FAW) was detected for the first time in Australia in early 2020 in northern Queensland. It migrated to Kununurra, Broome, and Carnarvon, and was detected in moth traps at Geraldton and Gingin later in the season. It is reported to feed on more than 350 host plant species, including cotton, maize, rice, sorghum, wheat, fruit and vegetable crops. In addition to cereals, canola and pulse crops, lucerne, and C4 grasses are potential hosts for this species.
Fall armyworm is a highly mobile moth, capable of long distance flights. It is a tropical to sub-tropical pest that does not do well in the cold, and is unlikely to be a serious pest of winter crops in Western Australia. However, it is possible that the moths may migrate large distances to grainbelt regions when temperatures warm up in spring.
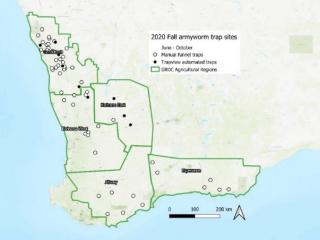
Over 70 pheromone moth traps were established in the WA grainbelt to detect migration of fall armyworm from northern sources, such as Carnarvon. A combination of manual bucket traps (Unitrap®) and automated moth traps (TrapView®) was monitored by DPIRD staff, grower groups and agronomists from June to October, until crops senesced.
Although fall armyworm moths were detected at Geraldton and Gingin in 2020, no moths or larvae were detected at grainbelt sites or from public reports of caterpillars via samples or images. Trap sites are presented in Figure1. At time of publication of this report, Carnarvon is the most southern point that fall armyworm caterpillars had been confirmed. A male (and suspected female) fall armyworm moth was captured at Northam in May 2021.
Russian wheat aphid
Russian wheat aphid is found in all major grain growing countries, where it is a pest of wheat and barley. The main concern regarding Russian wheat aphid is its potential to cause yield loss. Unlike other aphids, during feeding Russian wheat aphid injects toxins that retard growth, leading to yield loss. Crops are most at risk from Russian wheat aphid feeding damage after GS30 (start of stem elongation) until GS50 (start of head emergence).
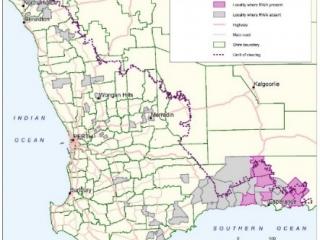
In August 2020, DPIRD initiated a Level 2 Incident, which ran for three months. DPIRD staff, growers, grower groups, and consultants conducted field surveillance of wheat and barley crops to determine the geographical distribution of the pest.
More than 120 sites were surveyed throughout the grainbelt however, Russian wheat aphid was found in 24 sites in the Esperance port zone only (Figure 2). Sites with Russian wheat aphid were located in low, medium and high rainfall areas, and on early and late sown barley and wheat crops. Surveys found notably fewer Russian wheat aphid in crops that had an insecticide seed dressing. Russian wheat aphid was present at levels of less than 1% of tillers, which is well below control thresholds.
Conclusions
Fall armyworm and Russian wheat aphid are new pests of grain, pasture, and horticulture crops in Western Australia. Proper identification, surveillance, and an integrated pest management approach will be key in managing these pests.
Growers should be vigilant and report suspect fall armyworm caterpillars, given their migratory ability of large distances over short periods of time.
Russian wheat aphid was not found outside of Esperance during 2020, but it is expected to migrate from the Esperance port zone. In WA, DPIRD will undertake surveillance in 2021 to determine the persistence of Russian wheat aphid in Esperance, and its presence in other areas of the WA grainbelt.
To view the full paper, refer to What is going on with fall armyworm and Russian wheat aphid in Western Australia?
Readers can view this presentation at the 2021 GRDC Grains Research Update Perth page. Find the Day 2, Session 14 recording.
For more information, contact DPIRD entomologists Dusty Severtson, on +61 (0)8 9690 2160 or Svetlana Micic on +61 (0)8 9892 8591.
Controlling redlegged earth mites using intensive spring grazing
Past research found that intensive grazing of pastures throughout spring could control redlegged earth mite (RLEM) as effectively as insecticides. However, the tactic has not been adopted by producers due to the impractical number of sheep required for months in one paddock.
Insecticide resistance in RLEM populations in parts of the grainbelt has renewed interest in alternative control methods, such as intensive spring grazing around the Timerite® period.
While any mixed farming enterprise producer could benefit from a spring grazing package aimed at controlling RLEM, the most significant benefits are expected to be experienced by producers in the medium to high rainfall zones with more pasture feed on offer (FOO), and larger RLEM populations.
In 2019, DPIRD research scientists, Svetlana Micic and Paul Sanford, worked with Fitzgerald Biosphere Group, Southern Dirt, Gillamii Centre, and associated farmers to conduct on-farm demonstrations at Boyup Brook, Cranbrook, and Kalgan. The demonstrations compared the effectiveness of intensive grazing for two, and four weeks in spring, around the Timerite® date, with an ungrazed control.
Boyup Brook results
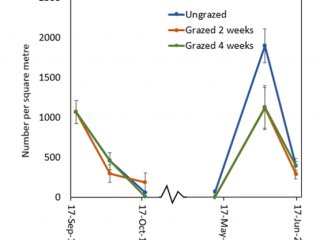
In 2019, the intensively grazed plots at Boyup Brook were maintained at about 2t DM/ha of dry matter (Figure 3). By the completion of two weeks’ grazing (2 Oct 2019), there were 95% fewer RLEM compared to the control (Figure 4). Two weeks after livestock had been removed from this treatment, the numbers of RLEM had increased five-fold (84%), whereas FOO only increased by 0.5t DM/ha (Figures 3, 4). Four weeks of grazing led to a 98% reduction in RLEM compared to the ungrazed control, and a fortnight later (6 November 2019) RLEM populations had crashed, indicating the population was undergoing summer diapause.
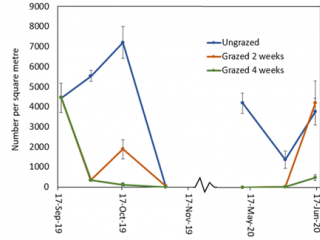
Kalgan results
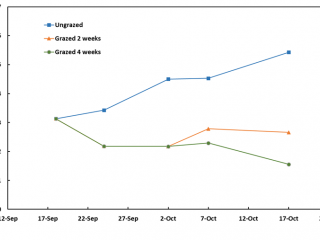
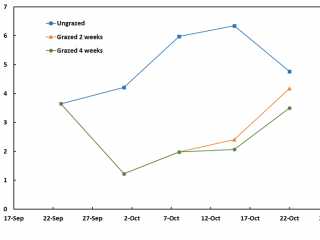
Within a week of imposing the intensive grazing treatment on pasture, FOO had fallen to 1.6t DM/ha however, by the end of the four-week grazing period, FOO had risen to 3t DM/ha due to late rain that lifted pasture growth rates (Figure 5).
Compared to the ungrazed control, grazing for two and four weeks resulted in a 90% reduction in RLEM numbers. When livestock was removed after two weeks’ grazing, RLEM numbers increased by 10% (Figure 6). By early November, RLEM numbers had decreased to less than 100 per square metre in the grazed treatments. When resampled in June 2020, the ungrazed treatment had three times as many mites as the grazed treatments.
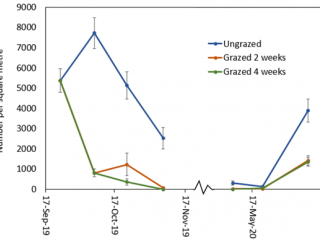
Cranbrook results
At the commencement of the demonstration, pasture FOO was low for a spring pasture, at 2.5t DM/ha. Grazing for two and four weeks only reduced FOO to about 2t DM/ha. At the end of this four-week period, FOO in the ungrazed treatment had increased by about 0.7t DM/ha, indicating the pasture growth rates were relatively low compared to the Boyup Brook and Kalgan sites (Figures 3, 5, 7).
Grazing did not cause a significant reduction in RLEM numbers as the population, unlike at the other sites, had declined in the ungrazed plot due to the dry conditions. By mid-October, RLEM numbers had crashed (Figure 8). By the end of May 2020, the ungrazed plot had 40% more mites than grazed plots. By mid-June, RLEM numbers across all treatments had dropped below 500 mites per square metre.
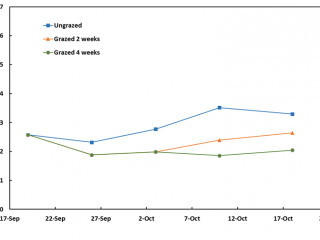
Conclusion
Intensive grazing in spring appears promising as a tactic for suppressing RLEM for the following season. However, it should only be used when the mite population is above 5000 per square metre, and FOO is more than 3t DM/ha. To achieve a significant reduction in mites, the pasture needs to be grazed to a FOO of 2t DM/ha or less for four weeks around the Timerite® period. Control measures for canola, such as seed dressings, may be required to protect the crop from RLEM damage.
To view the full paper, refer to Controlling redlegged earth mites using intensive spring grazing.
Readers can view this presentation at the 2021 GRDC Grains Research Update Perth page. Find the Day 2, Session 18 recording.
For more information, contact DPIRD entomologist Svetlana Micic on+61 (0)8 9892 8591.
Movement, breeding, baiting and biocontrol of Mediterranean snails
Molluscicidal baiting is an important component of integrated snail control but provides variable levels of control, despite high cost.
Efficient baiting must target adult snails before most reproduction occurs. Effective baiting to ensure snails encounter pellets requires snail movement, which must be predicted before application.
The reproductive cycles of three snail species were studied at four South Australian and four Western Australian locations between 2017 and 2020, for periods of 2 to 4.5 years. Target species were: vineyard snail (C. virgata) at three SA sites and one WA site; white Italian snail (T. pisana) at one SA site; and the small pointed snail (C. barbara) at three WA sites (Table 1).
Movement behaviour of snails was studied using time lapse video footage at 10 locations in SA and WA (seven sites in Table 1, with exception of Manoora, plus three other SA sites) between 2015 and 2020, for periods of 9 months to 4.5 years.
The research highlighted that baiting programs targeting C. virgata, T. pisana, and C. barbara should be concentrated during the autumn and early winter period, from approximately March to June, prior to most reproduction, to maximise cost-efficiency.
| Species | Study location | Study years | Breeding season average | Breeding season range | |
|---|---|---|---|---|---|
| Vineyard snail, Cernuella virgata | SA | Palmer | 2015 - 2018 | Mar to Sep | Feb/Mar to Jul/Oct |
| SA | Manoora | 2015, 2017, 2018 | Mar to Oct | Mar/Apr to Oct/Nov | |
| SA | Urania | 2018 - 2020 | Apr to Sep | Mar/May to Aug/Oct | |
| WA | Gairdner | 2017, 2018 | Mar to Oct | Feb/Mar to Oct/Nov | |
| 4 sites | 12 years | Mar to Sep | |||
| White Italian snail, Theba pisana | SA | Warooka | 2015 - 2018 | Feb to Jul | Jan/Feb to Jul/Aug |
| 1 site | 4 years | late Feb - late Jul | |||
| Small pointed snail, Cochlicella barbara | WA | Esperance Munglinup | 2018 | Jan to Sep | |
| WA | Esperance Condingup | 2017, 2018 | Mar to Sep | Feb/Apr to Sep/- | |
| WA | Woogenellup | 2017, 2018 | Mar to Nov | Mar/Apr to Nov/- | |
| 3 sites | 5 years | Mar to Oct | |||
In general, snails became increasingly responsive (moved) to increases in ground level relative humidity, from late summer through autumn. For simplicity, rule-of-thumb guidelines for snail movement with respect to relative humidity were generated from the data (Table 2).
| Species | Feb | Mar | Apr | May | Autumn |
|---|---|---|---|---|---|
| Vineyard snail | > 95% | > 90% | > 80-85% | > 85-95% | |
| Italian snail | > 90% | > 90% | > 85-90% | > 88% | |
| Small pointed snail | > 95% | > 95% | > 95% | > 95% | |
Introduced parasitoid fly Sarcophaga villeneuveana attacks two species of snail: the large pointed snail (Cochlicella acuta); and the small pointed or small conical snail (C. barbara). This strain of S. villeneuveana was sourced from the Montpellier region of France, and introduced into South Australia by SARDI and CSIRO between 2001 and 2004 for biocontrol of C. acuta.
Further research under laboratory conditions has found that a strain of S. villeneuveana from Morocco causes more parasitism than the strain from Montpellier in both species of snail. Prior to release of the Moroccan strain, a baseline survey of the current distribution and rate of parasitism of the Montpellier strain of S. villeneuveana was undertaken to determine if there had been a change in rate of parasitism, and spread.
It was discovered that introduced parasitoid fly, S. villeneuveana had successfully established on the southern Yorke Peninsula in South Australia, and is more likely to persist in areas where there is a florescence food source over summer. If the snail was present on an elevated subtrate over summer, and not at the base of plants or under refuges, parasitism increased to 11% for C.acuta and 13% for C. barabara.
To view the full paper, refer to Movement, breeding, baiting and biocontrol of Mediterranean snails.
For more information, contact Dr Kym Perry, SARDI on +61 (0)8 8429 0738 or Svetlana Micic, DPIRD on +61 (0)8 9892 8591.
Know what is in your chaff?
The three techniques most commonly employed in harvest weed seed control (HWSC) systems are: chaff dumping; chaff lining; and chaff tramlining.
The GRDC western region has the highest adoption of HWSC, with an estimated 67% of all farmers undertaking at least one HWSC strategy in 2014.
Chaff dumping is the collection of the chaff fraction using a cart towed behind the harvester. The chaff in the cart is dumped, usually in piles in the paddock, to be burnt, grazed, or left to decompose.
Chaff lining involves funnelling the chaff fraction of crop residue (containing weed seeds) into a confined row, directly behind the harvester, using a narrow chute. The chaff and weed seeds are left to decompose over time. To promote decomposition, the chaff lines need to be placed in the same location, year after year, by running the harvester on a controlled traffic system (CTF) system.
Chaff tramlining is a similar concept to chaff lining, but the chaff fraction is diverted through a chaff deck onto permanent wheel tracks in a CTF system. Wheel traffic creates a hostile environment that inhibits weed seed germination.
Recently, more growers have been opting to leave chaff in-situ to rot, whether in dumps or lines, rather than burning.
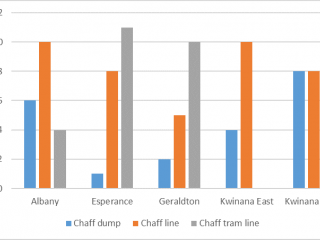
A surveillance project was conducted to better understand which invertebrate species were associated with HWSC systems in each of the five WA port zones: Albany, Esperance, Geraldton, and Kwinana East and West.
A total of 67 paddocks was surveyed from 2019 to 2020. An effort was made to identify a similar number of HSWC systems per port zone for this study however, some HWSC systems are under-represented in certain zones. For instance, chaff tramlining is more common in the Esperance port zone than in Kwinana port zones (Figure 9).
In each paddock, two methods (direct surveys and pitfall traps) were used to assess invertebrate numbers pre-seeding, and post-seeding. Direct surveys involved assessing chaff, and stand stubble, for invertebrates. Pitfall traps consisted of 250mL containers being placed in two rows and kept open for 7 days. There were 10 pitfall traps per row, spaced at least 10m apart. One row was placed adjacent and at a 5cm distance from chaff. The second row was placed parallel to the first row and 20m away from chaff.
Invertebrates were identified to species, and divided into functional groups of natural enemies (ie species that predate on pests, harvest weed seeds) or pests (ie species that are known to feed on germinating crops).
Direct sampling
Direct sampling was a found to be a poor method in looking for invertebrates in paddocks, with less than 1% of all surveys finding a single invertebrate in paddocks. Invertebrates were 80% more likely to be found in the chaff than in standing stubble.
Pitfall trapping
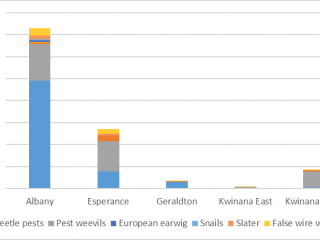
Unlike the direct sampling method, pitfall traps captured invertebrates in every paddock, however, the diversity of invertebrates captured varied between port zones. Pitfall traps located in the Albany port zone captured on average 70% more pests, and pitfall traps located in the Kwinana East port zone captured 70% more weed seed harvesting ants than pitfall traps in other port zones (Figures 10, 11).
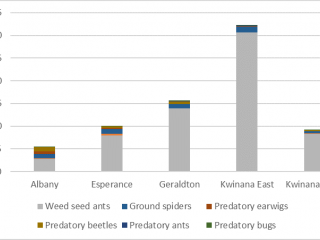
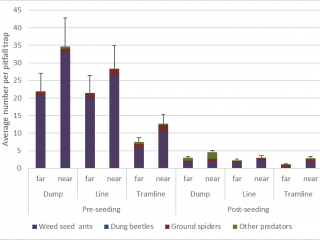
If the HWSC system is taken into consideration, pitfall traps adjacent to chaff dumps captured the most (40% more) natural enemies compared to pitfall traps located adjacent to chaff from lines or tramlines. The most abundant natural enemy was weed seed harvesting ants, comprising 90% of the average pitfall trap catch (Figure 12). However, after seeding, there was a 90% reduction in natural enemies caught in pitfall traps.
Pests such as slaters, snails, pest beetles, and European earwigs were 70% more likely to be found in pitfall traps next to chaff from lines or dumps, whereas desiantha weevil was more likely (40% more) to be captured in pitfall traps located in standing stubble (Figure 13). After seeding, pitfall trap catches of desiantha weevil, European earwig, and snails increased, suggesting that these pests are more likely to actively move after seeding has occurred.
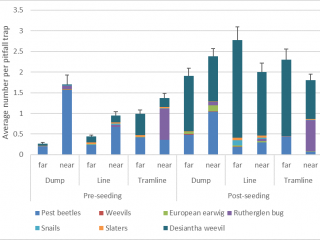
Conclusions
Both pests and natural enemies were found in association with chaff, and the species composition depended on the location of the paddock. Paddocks in the Albany port zone were more likely to have higher densities of pests, whereas paddocks located in the Kwinana East port zone were more likely to have beneficial insects, like weed seed ants.
Overall, chaff in tramlined paddocks had the least number of pests and beneficials, suggesting that the active movement of machinery over the lines may decrease the presence of invertebrates. However, pests such as European earwigs, slaters, and snails use chaff as a refuge. Control measures in a paddock could be targeted to areas of the paddock with chaff.
These surveys highlighted that actively looking for invertebrates in paddocks is difficult, but pitfall traps are a useful tool to determine if pests are present.
Readers can view this presentation at the 2021 GRDC Grains Research Update Perth page. Find the Day 2, Session 18 recording.
For more information, contact Svetlana Micic, DPIRD on +61 (0)8 9892 8591.