Fall armyworm update
DPIRD has conducted extensive surveillance for fall armyworm (FAW) throughout agricultural regions of WA since its introduction into Australia in early 2020. With regards to the WA grainbelt, to date trapping efforts have found that FAW is only established in Gingin. As part of the ongoing surveillance program run by DPIRD, FAW moths have been detected in traps near Geraldton, and Northam, but it has not been confirmed as established in these areas and no larvae were found.
DPIRD sent samples of FAW larvae from the Ord Valley Irrigation Area to the Insecticide Resistance Unit at New South Wales’ Department of Primary Industries (NSW DPI) for analysis. Larvae were tested for the presence of genes that are linked to resistance.
Genes that may lead to resistance to the Group 1 insecticides were found in all of the larvae tested, which means that the FAW population in Western Australia will have reduced susceptibility to the Group 1A and 1B insecticides (organophosphates and carbamates). For further information see DPIRD’s latest industry update
For more information on how to identify FAW and about DPIRD’s trapping efforts for this pest refer to DPIRD’s 2021 PestFax Issue 4 article Fall armyworm moths found in Northam
For more tips on identifying FAW refer to DPIRD’s:
- 2020 PestFax Issue 14 How to distinguish Fall armyworm caterpillars from other endemic caterpillars article
- Fall armyworm larval identification guide factsheet
- How to identify fall armyworm video.
For more information on FAW refer to:
- DPIRD’s Fall Armyworm in Western Australia page
- DPIRD’s Fall armyworm larval identification guide fact sheet
- GRDC’s Fall armyworm portal
For more information contact Dustin Severtson, Research scientist, Northam on +61(0)8 9690 2160 or +61 (0)427 196 656.
Article authors: Svetlana Micic (DPIRD Albany) and Dusty Severtson (DPIRD Northam).
Green peach aphid and virus update
- Widespread from Geraldton to Esperance
Green peach aphids (GPA) have recently been reported in canola crops ranging from the Geraldton to Esperance port zones.

Courtney Piesse (Agronomique Services) reports that GPA have caused crop loss in hot spots in canola paddocks in the Williams and Arthur River area. In some paddocks, GPA hot spots have corresponded with occurrence of radish, indicating GPA have moved from dying radish plants into the crop. The hot spots in the paddocks have been sprayed.

Plant virologist Benjamin Congdon (DPIRD) has found GPA present throughout the WA broad acre growing areas. Many of the GPA which have been tested were found to carry the Turnip yellows virus (TuYV). TuYV can decrease yield, and seed quality in canola if infection occurs before the rosette stage.
Once a crop has GPA present, it can be too late to spray the aphids to prevent TuYV, however spraying may still be required if the crop is not out-growing feeding damage. Trials conducted by DPIRD have found if GPA colonise crops before flowering, at the rosette stage or earlier and numbers are greater than 600 per leaf at 10% flowering, the affected plants will senesce earlier and have decreased yield. Spraying for GPA at early flowering when GPA were at 600 per leaf led to no yield loss occurring.
Dan Taylor (DKT Rural Agencies) reports that he has been starting to find GPA that have been infected with fungus in canola crops in the central agricultural region. Aphids infected by fungi are sluggish and have white/yellow to orange ‘fur’ covering their bodies. The fungus can readily spread throughout aphid colonies. The fungus can be more effective in decreasing aphid populations than chemical control. Conducive conditions occur when rainfall or dew allows continually moist conditions in the crop canopy and days are not too cold.
Growers are urged to check canola crops for fungal infections in aphids before deciding to invest in an insecticide spray.

GPA are known to have resistance to four different chemical groups – synthetic pyrethroids (for example, alpha-cypermethrin), organophosphates (for example, dimethoate), carbamates (for example, pirimicarb) and now, neonicotinoids. Neonicotinoids, such as imidacloprid, are applied as seed treatments in the grains industry and have become commonplace in canola pest management across Australia.
To reduce the risk of resistance to any insecticide group, it is important to rotate insecticides with different modes of action, avoid the use of broad-spectrum insecticides and apply appropriate insecticides only after careful monitoring and correct identification of species. Growers and advisors are encouraged to download and follow GRDC’s GPA Resistance Management Strategy to minimise the further development of insecticide resistance.
If you see aphids colonising canola crops, or plants that look symptomatic that you are concerned about, they can be tested for the presence of TuYV through the Department’s Diagnostic Laboratory Services – Pathology Services.
For insecticide recommendations, refer to DPIRD’s 2021 winter spring insecticide guide.
For previous aphid and TuYV findings and management information, refer to DPIRD’s:
- 2021 PestFax Issue 2 article Check emerging canola crops for aphids that may be carrying turnip yellows virus
- 2021 PestFax Issue 5 article Aphid and turnip yellows virus update
- Turnip yellows virus in canola: diagnosis and management page
- Turnip yellows virus early warning system page
- Aphid management in canola crops page.
For further information regarding TuYV contact Benjamin Congdon, Research Scientist, South Perth on +61 (0)8 9368 3499.
For further information on GPA, or other aphids, contact Svetlana Micic, Research Scientist, Albany on +61 (0)427 772 051.
Article authors: Benjamin Congdon (DPIRD South Perth) and Svetlana Micic (DPIRD Albany).
Russian wheat aphid update
- Williams
- Kellerberrin
- Narembeen
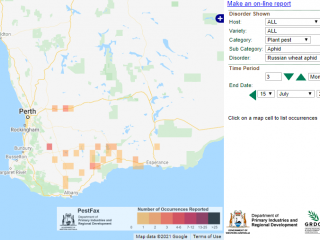
Russian wheat aphids (RWA) have now been recorded as far north as Kellerberrin. The PestFax map below displays reports of RWA received over the past three months by the PestFax team.
Courtney Piesse (Agronomique Services) has found on average 30 RWA per plant in ‘hot spots’ in an April sown barley paddock in Williams. The crop was at GS16 and did not have a seed dressing applied. The paddock was sprayed 5 weeks ago. However Courtney has found that plants in these hot spots have a prostrate habit, are still stunted in growth and remain at the 3 leaf stage. The surrounding plants have tillered and are at first node. No RWA were found in the paddock after spraying.
Reports of RWA continue to come in mainly from the southern areas of WA. In order to determine how far north RWA have spread, DPIRD will be arranging collections of barley grass from north of Kellerberrin. Barley grass is the preferred non-crop host for RWA, with researchers at SARDI reporting RWA presence in barley grass but not in the adjacent crop. If you would like to assist, please contact DPIRD entomologist Svetlana Micic on +61 (0)8 9892 8591.
RWA is expected to persist across the broad acre growing areas of WA. Crop yield loss is influenced by the percentage of tillers with RWA per paddock. A RWA threshold calculator is available on GRDC’s Russian wheat aphid page.
To read about previous RWA activity reported this season, refer to DPIRD’s 2021 PestFax Issue 9 article Russian wheat aphid activity update and feeding damage.
For more information on RWA, refer to the:
- DPIRD Russian wheat aphid: production pest page
- Cesar Australia Russian wheat aphid identification PestBites video
- GRDC Russian wheat aphid page
- GRDC Just how many Russian wheat aphids is too many podcast.
For more information on Russian wheat aphids, or other aphids, contact Research Scientist Svetlana Micic, Albany on +61 (0)8 9892 8591.
Article authors: Svetlana Micic (DPIRD Albany).
Native budworm caterpillars and moth trapping
- Northampton
- Dalwallinu
- Dowerin
- Grass Patch
- Mount Burdett
Plant pathologist Andrea Hills (DPIRD) reports finding low numbers of native budworm caterpillars around 20mm in size in an early flowering faba bean crop near Mount Burdett. Andrea has also found budworm caterpillars around 15mm in size on a chickpea crop near Grass Patch.
Volunteer farmers, agronomists and some DPIRD staff will commence weekly pheromone trapping for native budworm moths in the coming weeks as part of a program to monitor the potential risk of native budworm caterpillars to pulse and canola crops.
A couple of these volunteers have already installed their traps and reported some very low numbers of budworm moths this week at Dowerin (two moths) and Dalwallinu (two moths).
Two other budworm traps near Northampton have caught 47 and 91 moths over a two week period.
A mapped view of both the native budworm manual and automated trap captures is available at Cesar Australia’s MothTrapVisWA
More information on native budworm can be found in DPIRD’s 2021 PestFax Issue 9 article Native budworm are active.
Pesticide options for the control of native budworm can be found in DPIRD’s 2021 winter spring insecticide guide.
Detailed information on native budworm can be found at DPIRD’s Management and economic thresholds for native budworm.
For more information contact Alan Lord, Technical Officer, South Perth +61 (0)8 9368 3758 or +61 (0)409 689 468.
Article author: Alan Lord (DPIRD South Perth).
Net blotches found in barley crops and volunteers
Low levels of net form net blotch (NFNB) have been reported by AgWorld users and Plant pathologist Kithsiri Jayasena (DPIRD) on barley crops at:
- Green Range
- South Stirlings
- Kojaneerup South
- Amelup

Spot form net blotch (SFNB) has been found to been found at low levels on barley by DPIRD officers at the following locations:
- Gibson
- Worree
- Merredin
- Dalyup
- Beaumont
- Grass Patch
SFNB and NFNB are closely related fungal diseases that can significantly impact yield and grain quality of infected barley crops. NFNB infection and spread is favoured by wet conditions and it is most evident following periods of rainfall. It will cause the greatest yield loss in paddocks that are re-sown to barley without a break-crop as the fungus is carried from season to season on infected barley stubbles. Spores produced on stubble are spread by wind to initiate infections in new barley crops. Net blotch infections can occur following around six hours of leaf wetness at temperatures between 10-25°C. Seed infection is rare and is considered of minor importance in spreading disease.
Developing lesions of NFNB can be confused with spot form net blotch (SFNB). NFNB lesions appear on leaves as thin brown streaks or blotches that may enlarge up to several centimetres in length. Darker longitudinal and horizontal lines sometimes develop in the lesions, creating a net like appearance. Whereas SFNB lesions develop as small circular or oval dark brown spots with yellow edges.
The majority of varieties grown in WA are susceptible to SFNB and this disease occurs state-wide annually, particularly in continuous barley paddocks, but is most damaging in south coastal and neighbouring medium to high rainfall regions where it can have severe yield and quality effects.
Barley varieties that are susceptible to SFNB or NFNB, have not been treated with a registered seed dressing against net blotches (ie Systiva® or Uniform® in-furrow) and are sown into in a high risk situation (barley on barley) are particularly vulnerable. To see which barley varieties are susceptible to SFNB and NFNB refer to DPIRD’s 2021 WA Crop Sowing Guide - Barley
Growers with significantly higher levels of NFNB disease infection are also encouraged to collect leaf samples before applying fungicide and to send them to DPIRD to assist ongoing research into the pathotypes present in WA. Growers sending samples with suspected NFNB to the department can contact DPIRD technical officer Jason Bradley on +61 (0)447 864 707 or post leaves to Jason (in paper envelopes with variety and GPS/location information) at Locked Bag 4, Bentley Delivery Centre, WA 6983.
Timing of applications for optimal disease management
Applying a fungicide spray is necessary in medium to high rainfall regions where disease threatens crops with high yield and quality expectations, particularly if the barley crop has been sown into or adjacent to last year’s barley stubble. However, due to the presence of NFNB and SFNB populations that have reduced sensitivity or resistance to some fungicides, it is important to follow fungicide resistance management guidelines (see below).
The choice of a single-spray or two-spray strategy depends on the environment in which the crop is growing and the time of disease onset. In high rainfall environments or when disease is present from early growth stages it can be necessary to apply two sprays, such as at early stem elongation stage with a follow-up spray three to four weeks later.
In medium rainfall regions, consider one well timed spray between late stem elongation and early flag leaf emergence (Z33 - 39) to protect leaf 2 (flag-1). Under high disease pressure, best results can be obtained by using the maximum recommended rates. In low rainfall environments, fungicide application is most likely to result in a yield response to SFNB when the disease pressure is high and there is reasonable spring rainfall or stored soil moisture.
Application of a registered foliar fungicide prior to stem extension (during tillering) can reduce disease levels but is likely to require a follow-up fungicide later in the season.
Details on which foliar fungicide active ingredients are registered for SFNB and NFNB can be found at DPIRD's Registered foliar fungicides for cereals in Western Australia.
Managing net blotches and being mindful of resistance
At present, in the field SFNB is less sensitive to some active ingredients in the DMI group of fungicides in WA (eg. tebuconazole and propiconazole) and to the SDHI fungicides at locations in the central wheat belt, so applying these as single ingredient fungicides should be avoided, otherwise their use should be rotated with other groups. Fungicides in the QoI group (quinone outside inhibitors or ‘strobi’s’) are also registered to manage both net blotches in WA and have no recorded losses in efficacy against these diseases.
While DMI resistance in SFNB populations is more widely distributed compared to NFNB, NFNB pathogen populations less sensitive to DMI fungicide are present at multiple locations across the wheatbelt. In addition to this, the detections of “hybrid net blotch” (a mix between spot form and net form) which is highly resistant to DMI fungicides has made it important to adopt fungicide resistance management guidelines in WA.
For more information on fungicide resistance in WA net blotch populations refer to:
- DPIRD’s Management of barley net blotches in the face of fungicide resistance page
- CCDM’s The Spotlight article Resistant net blotch, Episode II:”Attack of the clones”
- GRDC’s Australian Fungicide Resistance Extension Network page.
If you suspect fungicide resistance in your paddock please contact the Curtin CCDM's fungicide resistance team at frg@curtin.edu.au .
To prevent further development of fungicide resistance it is recommended that growers only use fungicide if necessary, rotate fungicide modes of action, avoid using the same mode of action twice per season, use fungicide mixtures with more than one mode of action and use appropriate label rates. It is also recommended that tebuconazole is not used as a stand-alone product, in barley for any disease.
For further information on symptoms and management of blotches see DPIRD’s Managing spot type net blotch in continuous barley and Managing net-type net blotch of barley in Western Australia pages.
For more information contact Plant Pathologists Kithsiri Jayasena, Albany on +61 (0)8 9892 8477, Geoff Thomas, South Perth on +61 (0)8 9368 3262, Andrea Hills, Esperance on +61 (0)8 9083 1144 or Ciara Beard, Geraldton on +61 (0)8 9956 8504.
Article authors: Geoff Thomas (DPIRD South Perth), Kithsiri Jayasena (DPIRD Albany) and Andrea Hills (DPIRD Esperance).
Bacterial blight, Septoria avenae blotch and leaf rust are appearing in oats
Bacterial blight
- Muresk
Plant pathologist Kylie Chambers (DPIRD) has reported finding low levels of bacterial blight on several oat varieties in an oat trial at Muresk.
There are two bacterial blights that can affect oats in Western Australia, stripe blight (Pseudomonas syringae pv. striafaciens) and halo blight (Pseudomonas syringae pv. coronafaciens).
Stripe blight forms long, red-brown stripes on leaves during winter, which can join into blotches that can cause leaf collapse. Crop surveys over the last three seasons have found stripe blight to be the dominant form of bacterial blight in WA.
Halo blight causes pale green or yellow coloured, oval-shaped spots surrounded by a pale halo with a water-soaked appearance. As the disease develops, these spots turn brown and join together to form blotches. In recent seasons halo blight has been rarely reported in WA crops.
Both bacterial blights need moist conditions, such as cool showery weather, to spread the disease. Normally infection will stop spreading as canopies dry and crops can outgrow the infection in spring.
Spread of bacterial blight within oat crops is mainly by water movement such as rain splash or in some cases vehicle movement through a wet crop. The recent extended period of mild and wet conditions have been very favourable for expression and spread of bacterial blight.
The bacteria survive in crop stubble and soil debris for up to two years and can also be seed borne. Seed borne infection often results in hotspots of infection within paddocks.
Currently there are no crop protection chemicals suitable (or registered) to control this disease.
It is important not to confuse bacterial blight with fungal diseases like Septoria avenae or oat rusts, which can be suppressed by use of fungicides whereas bacterial blight is unaffected.
For more information see DPIRD’s Diagnosing stripe blight in oats and Diagnosing halo blight in oats pages.
Septoria avenae blotch
- Muresk
Plant pathologist Kylie Chambers (DPIRD) has also reported finding low levels of Septoria avenae blotch on several oat varieties in a DPIRD oat trial at Muresk.
Septoria avenae blotch symptoms can appear as brown to purple spots on leaves which develop into light and dark brown blotches, with dark brown centres. They are restricted and distinct at first but may enlarge and coalesce to cover most of the leaf.
Septoria avenae is the most prevalent oat disease in Western Australia and can impact both hay and grain yield and quality. The main source of infection is infected crop residue from previous seasons. The sexual stage of the fungus occurs on infected stubble and following rain in autumn and winter, they produce ascospores which are spread moderate distances by wind and are the source of infection in new crops. In consecutive oat rotations where stubble is not destroyed ascospores land on the new crop in large quantities resulting in the development of earlier and more severe outbreaks than in rotation crops. Secondary spread within the crop is by splash of spores formed in the lesions on leaves. Weather conditions this season appear to have been conducive for development of septoria infection.
Septoria avenae blotch can be minimised by avoiding continuous oat cropping but under high disease pressure, such as continuous oat crops, and in favourable environments, such as higher rainfall regions, foliar fungicides can be utilised to reduce disease severity and reduce potential losses to disease.
Trials carried out in 2014 by Ashton Gray (formerly ConsultAg) showed that in a disease favourable environment application of registered fungicide as a single spray at flag leaf or multiple sprays at stem extension and again at flag leaf could result in significant yield increase in the susceptible variety Bannister sown onto oat stubble.
Response to fungicide application will be determined by the level of Septoria disease present and by favourability of seasonal weather conditions. Trials conducted by Trent Butcher (ConsultAg) and Geoff Thomas (DPIRD) in 2019 found that fungicide application for Septoria did not improve grain yield or quality under low disease pressure scenarios. For more information, refer to the GRDC Update Paper Is there value in managing Septoria avenae blotch in oats in low disease pressure scenarios?
For a list of registered fungicides to use as foliar sprays visit DPIRD’s Registered foliar fungicides for cereals in Western Australia page.
For more information visit DPIRD’s Diagnosing Septoria avenae blotch in oats and Oats: leaf diseases pages.
Leaf rust
- Williams
Plant pathologist Geoff Thomas (DPIRD) reports finding trace levels of leaf rust in oat regrowth along the Albany Highway near Williams.
Oat leaf rust is also known as crown rust. The word 'crown' refers to the shape of a type of spore produced by this fungus and is not related to the disease symptoms. The disease is caused by the fungus Puccinia coronata var. avenae. The characteristic symptom is the development of round to oblong, orange to yellow pustules, primarily on leaves but also on stems and heads. The powdery spore masses in the pustules are readily dislodged. The pustule areas turn black with age.
For a list of registered fungicides to use as foliar sprays visit DPIRD’s Registered foliar fungicides for cereals in Western Australia page.
For more information refer to DPIRD’s Oats: leaf diseases page.
For more information on oat foliar diseases contact Plant pathologists Kylie Chambers, Northam on +61 (0)8 9690 2151 or Geoff Thomas, South Perth on +61 (0)8 9368 3262.
Article author: Kylie Chambers (DPIRD Northam).











