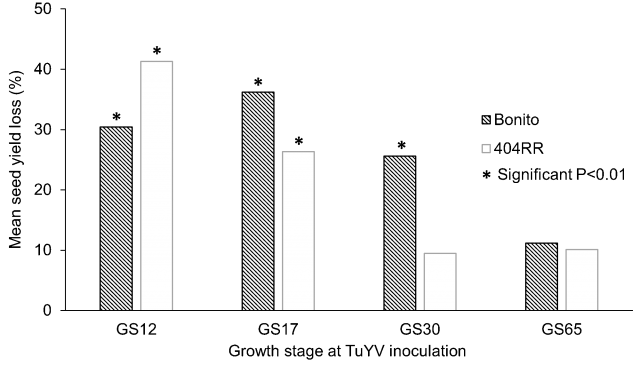The Department of Primary Industries and Regional Development's (DPIRD) Boosting Grains project 2019SP02, led by DPIRD research scientist Dr Ben Congdon in collaboration with Cesar Australia, is testing an early warning system for TuYV epidemics in Australian canola crops. By detecting migrating aphids carrying TuYV using yellow sticky traps and a rapid and sensitive RNA detection technique, this system may provide virus risk information to growers that enables proactive TuYV control strategies and prevent widespread TuYV infection and damage in canola crops.
What damage can TuYV do to a canola crop?
TuYV is a serious disease in canola, and can infect multiple Australian crops such as mustard, chickpea, lupin, lentil, faba bean, field pea, lucerne, medic and subterranean clover. Unfortunately, most Australian commercial canola varieties are highly susceptible.
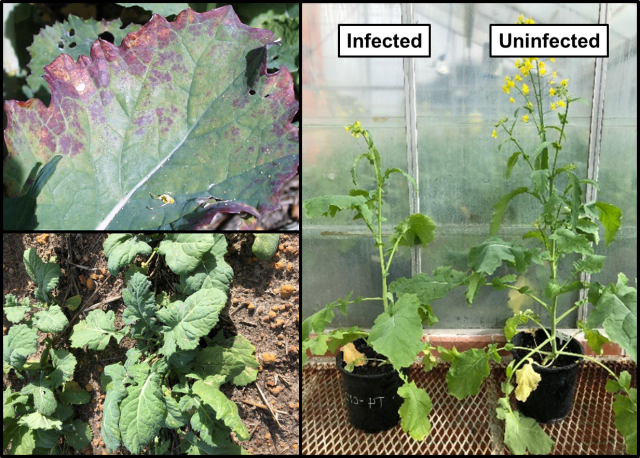
Since 2014, sporadic turnip yellows virus (TuYV) epidemics have caused widespread damage to Australian canola production with serious epidemics occurring in South Australia, Victoria and New South Wales (e.g. 2014) and Western Australia (e.g. 2018).
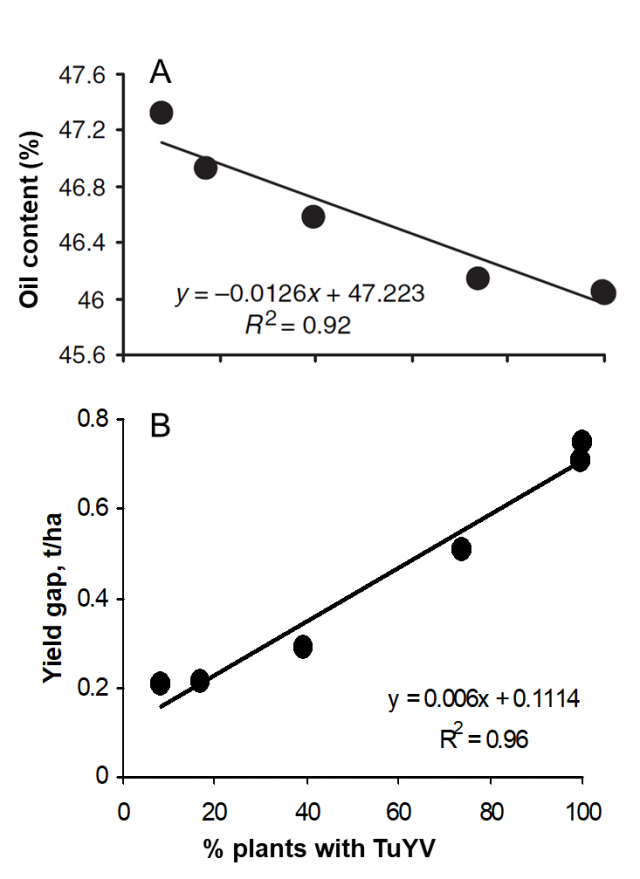
TuYV can halve yield, and severely compromise seed quality (Figure 2). However, time of infection is key (Figure 3), with heavier losses more likely if infection occurs during the rosette phase of canola up until stem elongation (GS30). Infection in canola crops after flowering is common across Australia but is unlikely to have a serious impact on seed yield and quality.
Why is TuYV difficult to control?
TuYV is spread by the green peach aphid (Myzus persicae) - a small but potent vector that can transmit TuYV through large areas of crop within a month or less. Effective control of green peach aphid is key in reducing TuYV infection.

Unfortunately, green peach aphid has evolved resistance to many insecticide chemicals. Effective chemicals currently available in Australia for control of green peach aphid are alarmingly limited. Overreliance and misuse of one of the remaining insecticides, sulfoxaflor (Transform®), could be leading to reduced sensitivity in some Australian green peach aphid populations (see Aphid and insecticide resistance management in grain crops).
Preliminary results also indicate that other biotic and abiotic factors could play an important role in the efficacy of canola seed treatments to control aphids and TuYV. Further research is underway by DPIRD and Cesar Australia to characterise these effects and its management implications.
Why is early warning particularly critical for TuYV control?
The best approach to controlling green peach aphid and TuYV is one which is proactive and integrated.
Such an approach includes control strategies like sowing into stubble, using a neonicotinoid seed treatment and delaying sowing to avoid peak autumn aphid flights, and the use of a systemic insecticide after sowing (Table 1).
The goal of these control strategies is to reduce spread during the vulnerable growth stages of the crop.
However, for several of these strategies to be optimally implemented, information is needed on the presence or absence of virus-carrying aphids in the environment. Without this information, control strategies are either not utilised or implemented reactively with no effect on the epidemic (e.g. late foliar insecticide application).
| Strategy | When | Effect |
| Controlling background weeds | At least two weeks pre-sowing | Reduces local aphid and virus reservoirs |
| Seed treatment | Pre-sowing | Protects seedlings from aphids and reduces virus spread early in crop |
| Sowing into stubble | Sowing | Reduces aphid landing rates and virus spread |
| Delay sowing | Sowing | Avoids exposing seedlings to peak aphid flights |
| Foliar insecticide | Post-emergence | Reduces aphid populations and may slow down virus spread |
Foliar insecticide applications are particularly time-sensitive due to the speed at which green peach aphid moves through a canola crop. It is common for sprays to occur too late to prevent virus spread. Currently, sulfoxaflor (Transform ®), flonicamid (MainMan ®) and afidopyropen (Versys ®) are the only active ingredients registered for effective control of green peach aphid in canola. For more information refer to Insecticide spray guides for crops in Western Australia.
Early warning could provide the grower with an opportunity to proactively intervene with an integrated control approach and make timely insecticide applications before significant yield damage can occur.
Conversely, if no virus-carrying aphids are detected, growers can continue with best agronomic approach for the crop and avoid wasting time and resources on foliar insecticide application. This has the added benefit of reducing impact on beneficial insects - which may be important later in the season for spring aphid control.
How does the early warning system work?
DPIRD has developed an early warning system for detecting migrating aphids carrying TuYV using yellow sticky traps and a rapid and sensitive RNA detection technique called ‘LAMP’ (Figure 4). This system is being trialled from 2019 to 2022 in Western Australia and in 2020 and 2021 in Victoria and New South Wales.
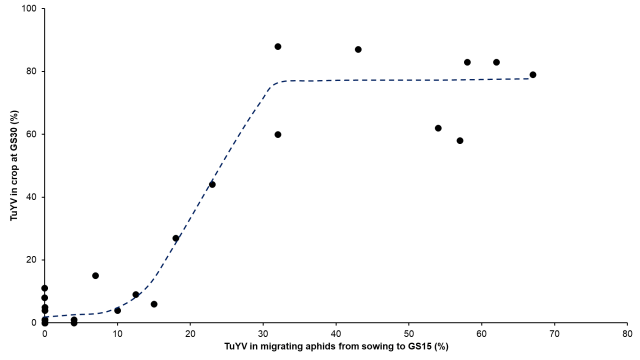
Using this system, TuYV is detected in migrating aphid vectors before a virus epidemic eventuates in the crop (Figure 5).

Sustained flights on virus-carrying aphids from around sowing time to the five leaf stage (GS15) has been demonstrated to be a strong predictor of the likelihood of a severe TuYV epidemic (Figure 6).
How to set up aphid traps
Materials required (provided)
- Two-sided yellow sticky traps
- Trap labels (with site and trap info)
- Sandwich ties
- Electrical tape
- Drinking straws
- Box or ziplock bags for packaging sticky traps
Firstly, please collect the following site information
- Location/GPS
- Canola variety sown
- Sowing date
- Insecticide seed treatment: nil, Gaucho, Cruiser Opti or Poncho Plus
- Background weeds e.g. wild radish
- Stubble retained: No or Yes (low, medium, heavy)
Setting up the traps - every two to four weeks beginning around sowing time until stem elongation
- Select a fenceline facing prevailing wind direction and/or near a potential virus reservoir e.g. volunteer canola, wild radish.
- Fix the sticky trap to top of the fenceline using sandwich ties (at least 1m above ground level) so that it cannot flap around in the wind, as shown below. Ensure the top edge of the punch hole is reinforced with electrical tape to avoid ripping in windy or wet weather. Set up two to three traps 50-100m apart at each site.
- Collect trap after 2 to 4 weeks and ensure trap details are filled out on the label e.g. date in, date out, crop growth stage and paddock I.D. Attach a fresh set of sticky traps to fenceline.
- When sending traps, it is important to minimise contact between the sticky glue and other surfaces when sending to avoid damaging aphids. Placing drinking straws on the sticky face can help maintain separation between traps when packing them.
- Record if and when (e.g. five leaf stage) the grower sprays Transform or any other insecticides applied to the crop.
Send traps to:
Ben Congdon,
3 Baron-Hay Court
Kensington WA 6151
Taking leaf samples
- Take the youngest leaf of 100 plants evenly across the sampling pattern. Begin sampling from the fenceline at the first trap to ~100 metres diagonally into the crop, then diagonally back to the fence line at the second trap, and so on in a ‘W’ pattern if three traps set up or a ‘V’ pattern for two traps (see above).
- Put leaf samples into plastic ziplock bag.
- Write paddock I.D. on bag using a marker pen.
- Send samples to:
Ben Congdon,
3 Baron-Hay Court
Kensington WA 6151
Acknowledgements
This work is supported by DPIRD Boosting Grains Science Partnerships project 2019SP02 in collaboration with Cesar Australia.